Combined inactivate vaccine for haemophilus parasuis disease and streptococcus suis disease and preparation method for same
A technology of haemophilus porcine disease and double inactivated vaccine, which is applied in the direction of antibacterial drugs, pharmaceutical formulas, bacterial antigen components, etc., can solve the problem of inability to know whether the antigen inactivation is complete, large vaccine side effects, inflammation or swelling And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of Haemophilus parasuis, Streptococcus suis dual inactivated vaccine (JS strain+ZJ strain+SC strain+Streptococcus equi zooepidemic subspecies ATCC35246 strain)
[0060] 1. Preparation of strains for production
[0061] Haemophilus parasuis serotype 4 JS strain, type 5 ZJ strain, Streptococcus suis type 2 SC strain, Streptococcus equi subsp. Serum type 4 JS strain, type 5 ZJ strain, Streptococcus suis type 2 SC strain, Streptococcus equi subspecies zooepidemic ATCC35246 strain for production.
[0062] (1) Primary seed propagation
[0063] First-class seed propagation was carried out on Haemophilus parasuis serotype 4 JS strain, type 5 ZJ strain, Streptococcus suis type 2 SC strain, and Streptococcus equi subspecies zooepidemic ATCC35246 strain. Streak and inoculate the freeze-dried strains of Haemophilus parasuis serotype 4 JS strain and type 5 ZJ strain on the TSA / NAD plate, culture at 37°C for 18 hours, select colonies that meet the requirem...
Embodiment 2
[0098] Embodiment 2: safety research
[0099] (1) Safety test of the vaccine on Balb / C mice
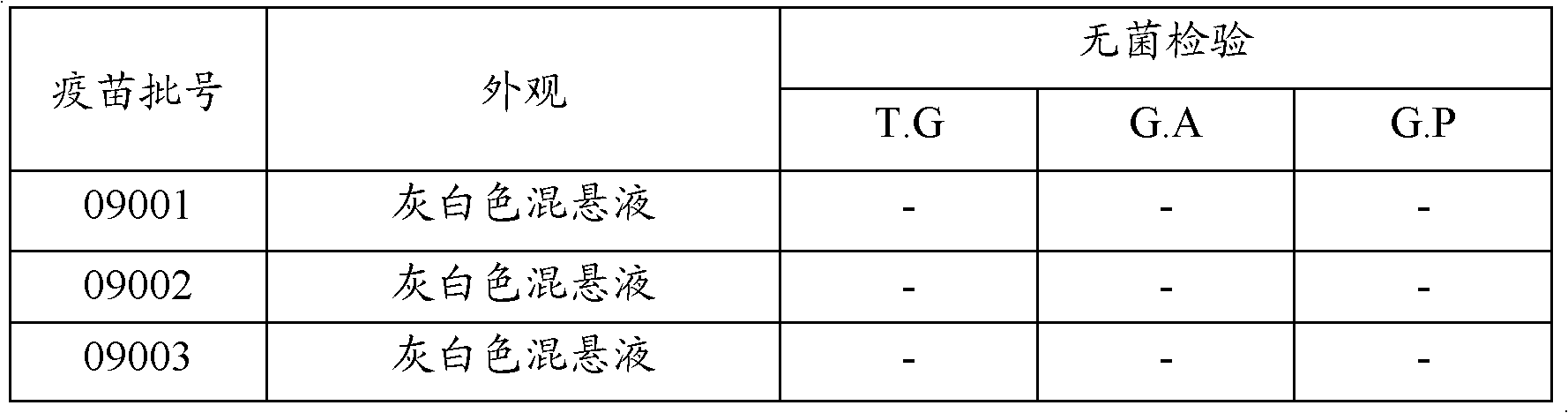
[0100] Three batches of prepared vaccines (batch numbers 09001, 09002, and 09003) were inoculated into 18-22 g Balb / C mice respectively, each subcutaneously injected with 0.4 ml, and observed for 14 days. The results are shown in Table 2. All Balb / C mice had no localized Responsive and all alive.
[0101] Table 2 vaccine is to the safety test result of Balb / C mouse
[0102] Vaccine batch Number of injections number of deaths local reactions only Pass rate 09001 10 0 0 5 / 5 09002 10 0 0 5 / 5 09003 10 0 0 5 / 5 control group 10 0 0 5 / 5
[0103] (2) Safety test of vaccine on piglets
[0104] Three batches of prepared vaccines (batch numbers 09001, 09002, and 09003) were injected intramuscularly into 5 30-day-old healthy susceptible pigs, each with 4 mL. There was no local reaction within 14 days a...
Embodiment 3
[0108] Embodiment 3: Efficacy test (tested in our company's experimental animal room)
[0109] (1) Vaccine efficacy test on Balb / C mice
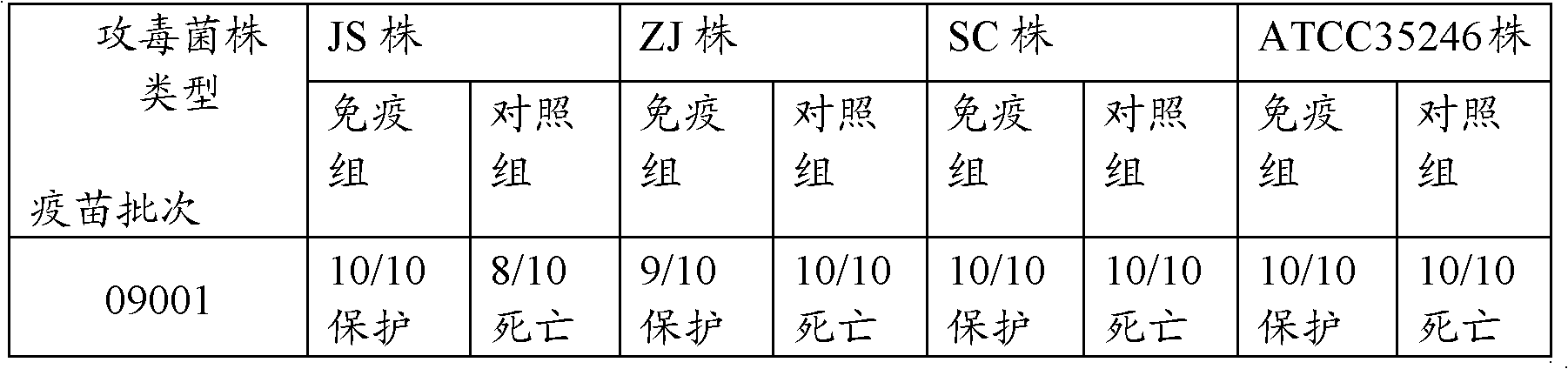
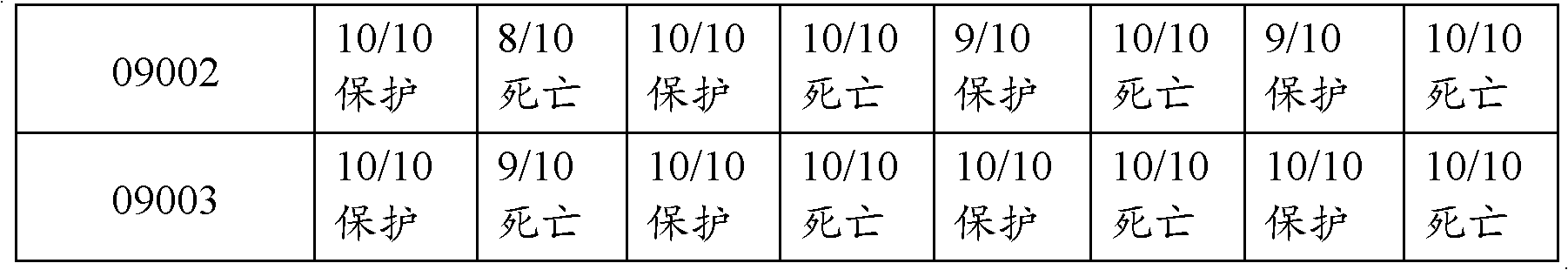
[0110] Balb / C mice weighing 18 to 22 g were subcutaneously inoculated with 0.1 mL of dual inactivated vaccine, and divided into 3 groups 28 days after immunization, together with the control group of 10 mice in each group. Inject 5LD 50 (The bacterial content is 4.0×10 9 CFU), the type 5 ZJ strain was injected intraperitoneally with 5LD 50 (The bacterial content is 2.5×10 9 CFU); Streptococcus suis type 2 SC strain subcutaneous injection 2LD 50 (The bacterial content is 1.0×10 2 CFU), Streptococcus suis group C Streptococcus equi subspecies zooepidemic ATCC35246 subcutaneous injection 5LD 50 (The bacterial content is 4.0×10 2 CFU) another 10 were set as blank control, observed for 14 days, and the test results are shown in Table 4.
[0111] Table 4 vaccine is to Balb / C mouse effect test result
[0112]
[0113]
[0114] (2) Pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com