Anti-porcine pseudorabies and swine flu vaccine composition and application thereof
A technology of vaccine composition and porcine pseudorabies virus, applied in antiviral agents, antibody medical components, medical preparations containing active ingredients, etc., can solve poor cross-protection, reduced vaccine immune effect, swine influenza virus hemagglutinin problems such as large differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of anti-porcine pseudorabies, swine flu vaccine composition
[0054] 1. Preparation of porcine pseudorabies virus antigen
[0055] Spinner bottle cell culture method. The porcine pseudorabies virus gene deletion strain (SA215 strain, TK - / gE - / gI - Three-gene deletion, disclosed in Chinese patent CN101186902, the strain was deposited in CCTCC with the preservation number V200002) was properly diluted with virus diluent (serum-free DMEM medium), and inoculated on monolayer ST cells according to M.O.I=0.1 (purchased from CCTCC, No. GDC0060), gently rotate the cell flask for 2 weeks, adsorb at 37°C for 30min, add DMEM cell maintenance solution containing 3% (v / v) calf serum, and rotate at 37°C (10-12 rpm / hours) for cultivation. Observe 1 to 2 times a day, the cells grow well, culture at 37°C for 2 to 5 days, harvest the cells and cell fluid, freeze and thaw 3 times, filter the virus fluid with a hollow fiber filter column (pore size 1....
Embodiment 2
[0069] Embodiment 2: Anti-porcine pseudorabies prepared by different lyoprotectants, swine influenza vaccine composition preservation and transportation comparison
[0070] 1. Materials
[0071] The anti-porcine pseudorabies and swine influenza vaccine compositions prepared by the optimized heat-resistant lyoprotectant are selected from V4 and V5 vaccines in Example 1; prior art configuration (5% lactose containing V / V, 10% skimmed milk aqueous solution ) prepared anti-pseudorabies and swine influenza vaccine compositions are V6 and V7. The antigen components of V6 and V4 are the same, only the lyoprotectant is different; the antigen components of V7 and V5 are the same, only the lyoprotectant is different.
[0072] 2. Experimental design
[0073] Vaccine compositions (V4, V5, V6 and V7) prepared with different lyoprotectants were sampled and inspected 10 days after they were placed at 37°C to determine the content of porcine pseudorabies virus in the vaccine composition, an...
Embodiment 3
[0078] Embodiment 3: Comparison of the production of PR antibodies after piglets are immunized with anti-pseudorabies and swine influenza vaccine compositions
[0079] 1. Materials
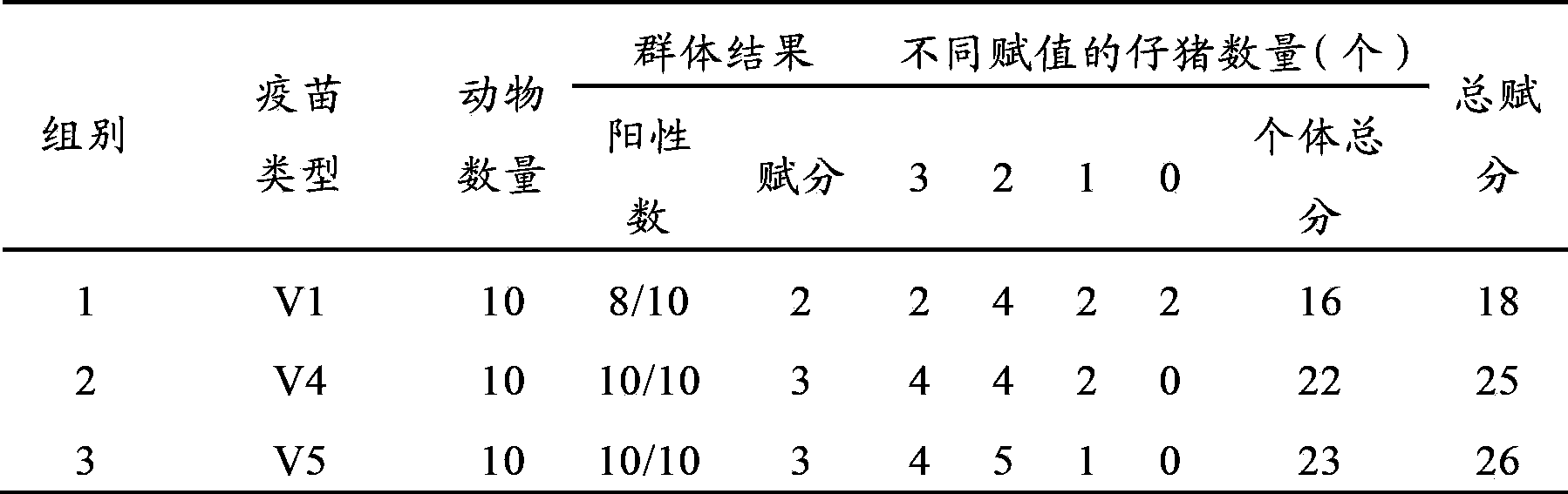
[0080] The anti-pseudorabies and swine influenza vaccine composition selects the V4 and V5 vaccines in Example 1; the porcine pseudorabies single vaccine selects the V1 vaccine in Example 1.
[0081] 2. Design of animal experiments
[0082] Select 30 21-day-old piglets and divide them into 3 groups, 10 pigs / group. In the first group, each pig is injected with the neck intramuscular injection of porcine pseudorabies single vaccine V12.0ml (1 dose / head), and in the second and third groups Each pig was intramuscularly injected with anti-porcine pseudorabies and swine influenza vaccine composition V4 and V5 2.0ml (1 portion / head). Twenty-eight days after immunization, the serum of piglets in each group was collected, and the antibody titer of porcine pseudorabies virus was determined by ELISA method...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com