Zika-virus-and-yellow-fever-virus combined inactivated vaccine
A yellow fever virus, Zika virus technology, applied in the direction of antiviral agents, virus/phage, virus antigen components, etc., can solve the problems of side reactions, no joint inactivated vaccine development of yellow fever virus and Zika virus, etc. Low cost, reduced vaccination times, and the effect of disease prevention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Yellow fever virus 17D spot-picking and adaptive culture in Vero cells
[0028] In order to make the yellow fever virus 17D adapt to the growth on Vero cells better, the live attenuated vaccine of yellow fever virus was used to pick spot culture on Vero cells. The lyophilized live attenuated yellow fever virus 17D vaccine was restored to its original volume with water for injection. According to the virus titer of the known vaccine, the virus was serially diluted with serum-free 199 medium. Inoculate each dilution of the virus into a 6-well plate that has formed a monolayer of cells, inoculate 0.4ml per well, and inoculate at 37°C, 5% CO 2 Adsorb in the incubator for 1 hour, and then add serum-free carboxymethylcellulose working solution. After culturing for a period of time, select culture wells with less than 3 plaques under a microscope. The plaques were sucked up with a pipette and seeded on a monolayer of Vero cells for culture. When the cytopathic ef...
Embodiment 2
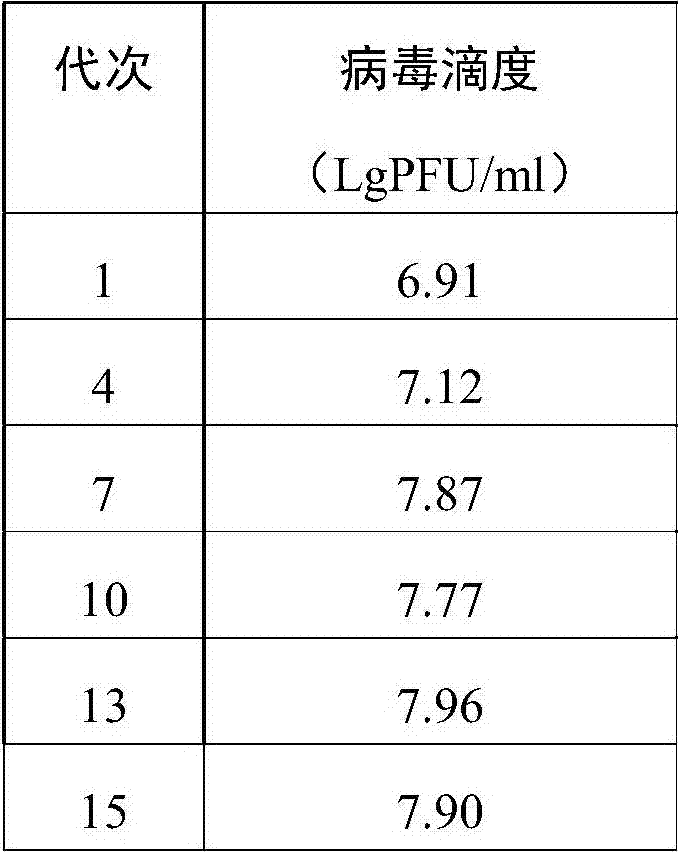
[0030] The titer and immunogenicity comparison of embodiment 2 yellow fever virus 17D in different passages on Vero cells
[0031] 1. Select the 1st, 4th, 7th, 10th, 13th, and 15th generations of adaptive passage on Vero cells to carry out virus titration test. Use the 199 cell maintenance liquid to serially dilute the virus, select 3 virus liquids with appropriate dilutions, and inoculate them into a 6-well plate in which the cells have formed a monolayer. Inoculate 2 wells for each dilution of virus, and set up 2 wells of cell control. At 37°C, 5% CO 2 Adsorb in the incubator for 1 hour, then add 4ml of carboxymethylcellulose solution to each well, and continue culturing in the incubator. After 6 days, take out the culture plate, discard the cover, add 1% crystal violet staining solution for staining, rinse with tap water, and dry in the air. PFU was calculated by counting plaques in wells with fewer than 30 plaques. The unit of virus titer is LgPFU / ml. Virus titer resu...
Embodiment 3
[0045] The cultivation optimization of embodiment 3 yellow fever virus 17D on Vero cells
[0046] For determining the optimal culture condition of yellow fever virus on Vero cell, inventor adopts JMP10 software to carry out experiment design according to inoculation mode, inoculation MOI, culture temperature and harvest time. The levels of each factor are as follows, the inoculation method is separate species and mixed species, the inoculation MOI is 1, 0.1, 0.01PFU / ml, the virus culture temperature is 33-37°C, and the harvest time is 25%, 50%, 75% of lesions, 100%. The optimal experimental conditions were determined by using the results of the virus titer of yellow fever virus. The experimental design and results are shown in Table 4:
[0047] Table 4 Yellow fever virus culture condition optimization result
[0048] temperature(°C)
[0049] Calculated by JMP10, CPE is a key process parameter, and the most suitable harvest is between 60-85% cytopathic. There was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com