Vaccine composition and preparation method against swine mycoplasma pneumonia and infectious pleuropneumonia
A technology of mycoplasma pneumonia and vaccine composition, which is applied in the direction of bacterial antigen components, antibody medical components, antibacterial drugs, etc., and can solve the problem of dual combination vaccine and drug prevention and control costs that can simultaneously prevent and treat mycoplasma pneumonia and porcine infectious pleuropneumonia increase, economic loss of aquaculture and other issues, to achieve the effect of low immunization cost, strong practicability, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Isolation and identification of Mycoplasma hyopneumoniae HN0613
[0048] 1. Materials and methods
[0049] (1) Materials
[0050] ① source of disease material
[0051] In 2006, 16 suspected lungs with mycoplasma swine pneumonia were collected from various places in Henan Province.
[0052] ② Culture medium
[0053] Mycoplasma hyopneumoniae Friis medium was purchased from BD Company; pig serum was purchased from GIBCO Company; phenol red indicator was purchased from American AMRESCO Company.
[0054] ③ Reagent
[0055] Biochemical reagents and chemical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd.
[0056] Bacterial Genomic DNA Extraction Kit was purchased from TIANGEN Company, and Dieter's Staining Kit was purchased from Chongqing Pangtong Medical Instrument Co., Ltd. Biochemical identification reagents were purchased from Hangzhou Tianhe Microbiological Reagent Co., Ltd. ④ Negative and positive serum
[0057] Rabbit anti-Mycoplasm...
Embodiment 2
[0093] Embodiment 2: Identification of the isolation of serum type 1 LC strain, type 5 YC strain, and type 7 YS strain
[0094] 1. Materials and Methods
[0095] (1) Disease materials to be tested
[0096] The disease materials of pigs with suspected pleuropneumonia were collected from 10 pig farms in Henan, Hubei and other provinces and cities.
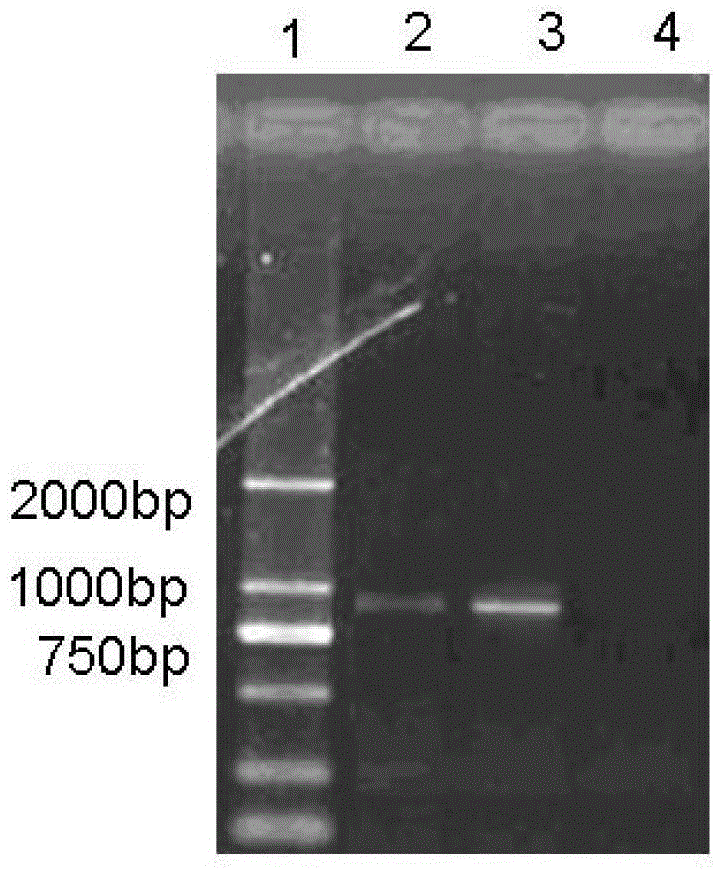
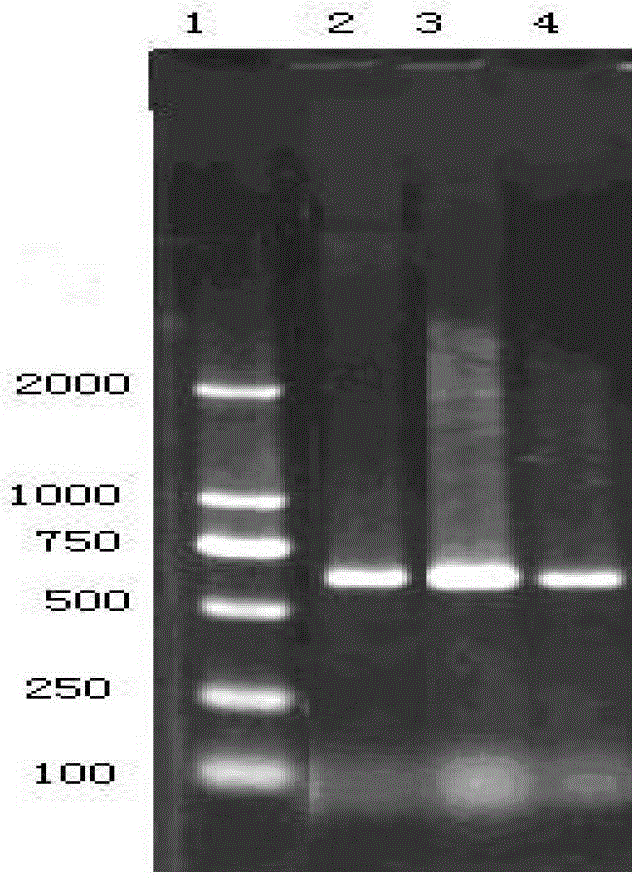
[0097] (2) PCR primer sequence
[0098] PF-5'CCGACTTTTAAATCCGT3', PR-5'GAACAGTTGTTCGCTAA3'
[0099] (3) Mice
[0100] Bought from Animal Experiment Center of Henan Province.
[0101] (4) Isolation and purification of pathogenic bacteria and liquid culture
[0102] Aseptically take lungs, throat tonsils and other tissues of dying pigs, inoculate them on TSA (containing 1wt% NAD) agar medium, place in 10% CO 2 After culturing at 37°C for 24-36 hours, a typical single colony was selected for purification. The purified single colonies were selected and inoculated in TSB (containing 1wt% NAD) liquid culture medium, and cultured on ...
Embodiment 3
[0128] Example 3: Preparation of Mycoplasma hyopneumoniae antigen and Actinobacillus pleuropneumoniae antigen
[0129] 1. Source of bacteria (virus) strains
[0130] The selected Actinobacillus pleuropneumoniae strains are: the preservation number of the serum type 1 LC strain is CCTCC M2011458; the preservation number of the serum type 5 YC strain is CCTCC M2011459; the preservation number of the serum type 7 YS strain is CCTCC M2011460. The date of deposit is December 9, 2011.
[0131] The selected Mycoplasma hyopneumoniae is HN0613, and the preservation number is CCTCC No. M2012230. The date of deposit is June 13, 2012.
[0132] 2. Preparation and inspection of vaccine semi-finished products
[0133] (1) Preparation of seeds for production
[0134] ① Actinobacillus pleuropneumoniae:
[0135] Propagation of primary seeds
[0136] Streak and inoculate LC, YC, and YS strains of freeze-dried strains on TSA (soybean casein agar) plates respectively, in 5-10% CO 2 Cultiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com