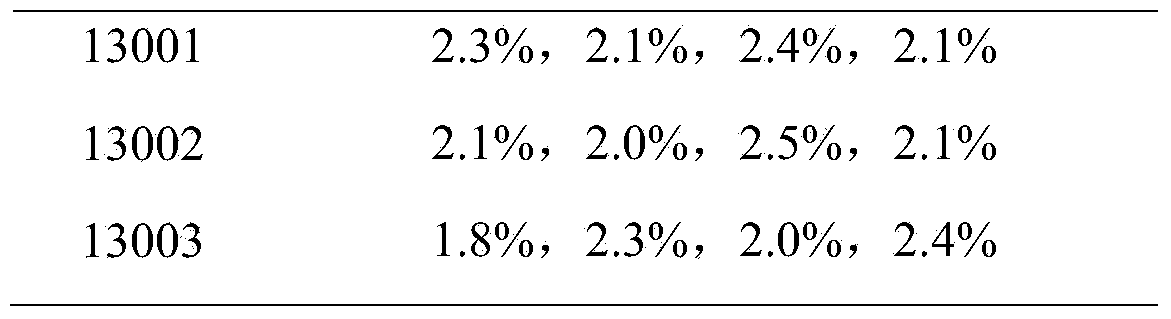Porcine circovirus type 2, porcine reproductive and respiratory syndrome bivalent vaccine and preparation method thereof
A technology for porcine circovirus and respiratory syndrome, applied in antiviral agents, viral antigen components, pharmaceutical formulations, etc., can solve the problems of immune antigen component interference, time-consuming and labor-intensive, and inconvenient to use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] ——Preparation of porcine circovirus type 2 and porcine reproductive and respiratory syndrome dual vaccine
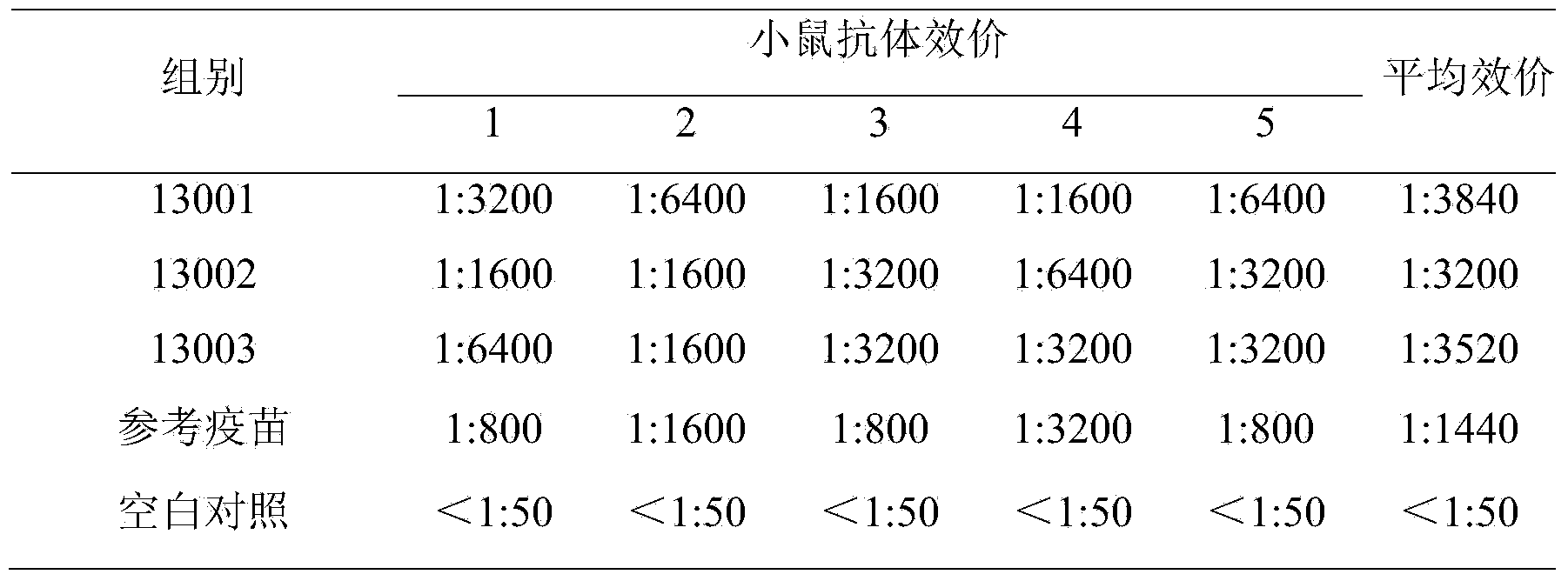
[0075] Taking porcine circovirus type 2 and porcine reproductive and respiratory syndrome dual vaccine batches 13001, 13002, and 13003 batches as examples
[0076] 1. Preparation and testing of porcine circovirus type 2 antigen solution
[0077] (1) PCV2SH strain (CGMCC No.2389, provided by Jiang Ping, Nanjing Agricultural University) kind of virus is inoculated to the PK15-B1 cell (provided by Jiang Ping, Nanjing Agricultural University) by (V / V) 3% to grow into a good monolayer 4% calf serum DMEM cell maintenance solution (V / V) was added to the bottle provided by the professor, and cultured at 37°C. Harvest after 4 days of culture, freeze and thaw three times at -20°C, harvest the cell culture, and take a small amount of samples for sterility testing and determination of virus content. After harvesting, the virus liquid was stored at -20°C.
[0078] (2) Steri...
Embodiment 2
[0101] ——Examination of porcine circovirus type 2 and porcine reproductive and respiratory syndrome dual vaccine
[0102] Taking porcine circovirus type 2 and porcine reproductive and respiratory syndrome dual vaccine batches 13001, 13002, and 13003 batches as examples
[0103] 1. Trait test
[0104] The properties of the three batches of dual vaccine prepared by the above method were tested, and the vaccine was a light yellow spongy loose mass, which was easy to separate from the bottle wall and dissolved quickly after adding the vaccine diluent.
[0105] 2. Sterility test, mycoplasma test
[0106] The three batches of vaccines were tested according to the method in the appendix of "Chinese Veterinary Pharmacopoeia", and the three batches of vaccines were all sterile and free of mycoplasma growth.
[0107] 3. Safety inspection
[0108] Dilute three batches of dual vaccine with vaccine diluent to contain 10 piglets per milliliter, intramuscularly inject 3 healthy piglets wi...
Embodiment 3
[0131] - Sterility test of vaccine diluent
[0132] Get 3 batches of vaccine diluents and carry out sterility test according to the appendix of "Chinese Veterinary Pharmacopoeia", and the test result shows aseptic growth in the vaccine diluents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com