Preparation method of C21-36 alicyclic diisocyanate and use
A technology of diisocyanate and alicyclic diisocyanate, which is applied in the field of preparation of C21-36 alicyclic diisocyanate, can solve the problems of harsh process conditions, easy occurrence of side reactions, and difficulty in industrialization, achieves environmental friendliness, and is conducive to industrial popularization and application. , the effect of less production difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of unsaturated cycloaliphatic dibasic acid (raw material A)
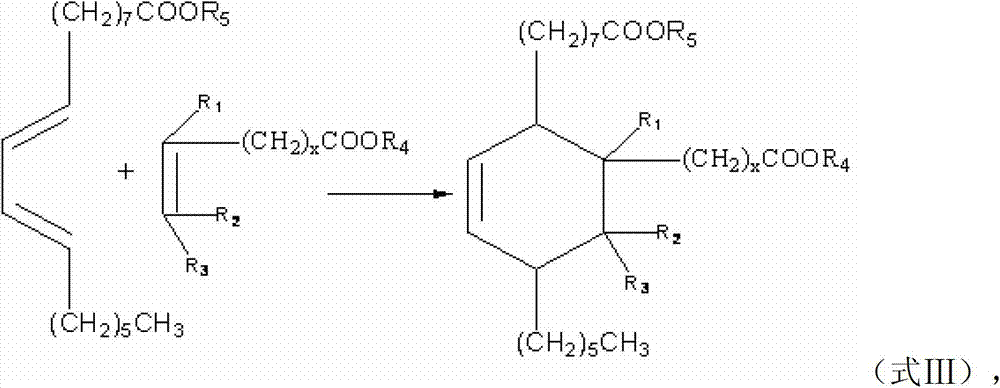
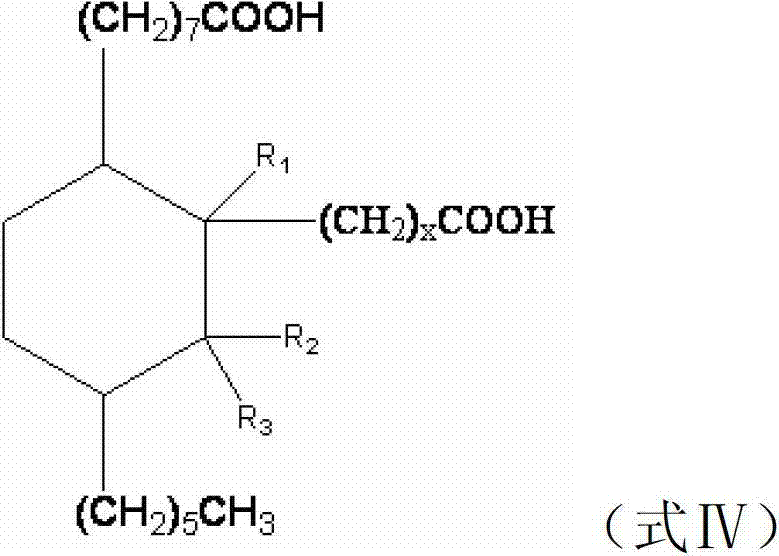
[0043] Add 1.7mol dehydrated ricinoleic acid ester, 1.7mol fatty acid methyl ester containing double bonds and 1.5g of hydroquinone as a polymerization inhibitor to a 2000mL pressure reactor, replace the air in the kettle with nitrogen, heat up to 160-180°C, and react The crude dibasic acid methyl ester was obtained in 3.5 hours. The above crude product was heated and refluxed at 80-90° C. for 4 hours with 680 mL of 10% sodium hydroxide solution in mass fraction, and acidified with hydrochloric acid with a mass fraction of 10%-15%. Saturated dibasic acid is distilled under reduced pressure to obtain a pure product, which is used as the basic raw material for preparing unsaturated alicyclic diisocyanate in subsequent examples, and its structural formula is shown in formula A:
[0044]
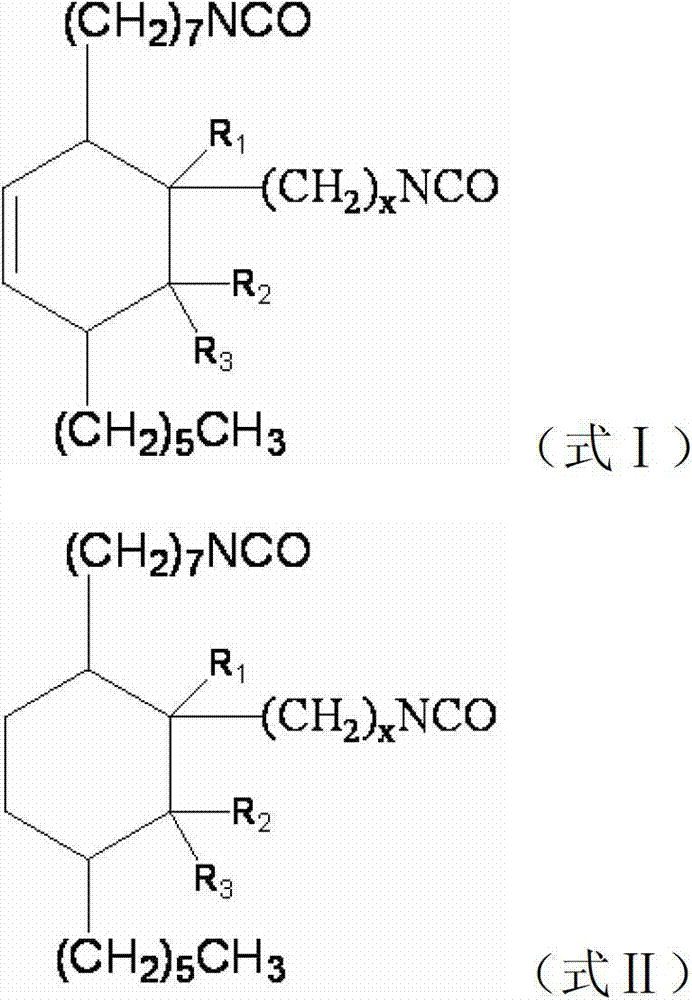
[0045] In the formula, x=1~7, R 1 , R 2 , R 3 for H atom or C 1-8 straight-chain or branched...
Embodiment 2
[0046] Embodiment 2: Preparation of unsaturated cycloaliphatic dibasic acid (raw material A)
[0047] The difference between this example and Example 1 is that the raw materials for preparing unsaturated alicyclic dibasic acids are different. In Example 1, esters are used to prepare acids, and in this example, acids are used to prepare acids.
[0048] Add 1.7mol of dehydrated ricinoleic acid and 1.7mol of double bond-containing fatty acid into a 2000mL pressure reactor, replace the air in the kettle with nitrogen, heat up to 160-180°C, react for 3.5 hours to obtain crude dibasic acid, and distill under reduced pressure to obtain pure As the basic raw material for preparing unsaturated cycloaliphatic diisocyanate in subsequent examples, its structural formula is shown in formula A.
Embodiment 3
[0049] Embodiment 3: Preparation of saturated alicyclic dibasic acid (raw material B)
[0050] Add the unsaturated aliphatic dibasic acid prepared in step embodiment 1 or embodiment 2 to another 2 liters of autoclave, add 500g methanol and 30g Pd (5%) / activated carbon catalyst (wet: dry weight 15g, 0.75g Pd), vacuumize, nitrogen replacement, hydrogen replacement respectively. The reaction mixture was stirred at 160-170° C., 1.5-2.0 Mp for about 7.5 hours, until the hydrogen absorption was complete, and the stirring was stopped. The material was filtered off under nitrogen pressure, leaving the catalyst in the filter. Use 500g of methanol to backwash the catalyst into the reactor, then add another part of the same unsaturated aliphatic dibasic acid, repeat the hydrogenation, the catalyst activity does not decrease, carry out the hydrogenation 5 times in the same way, and use the catalyst repeatedly for 4 Next, the obtained reaction mixture (colorless and transparent) is disti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com