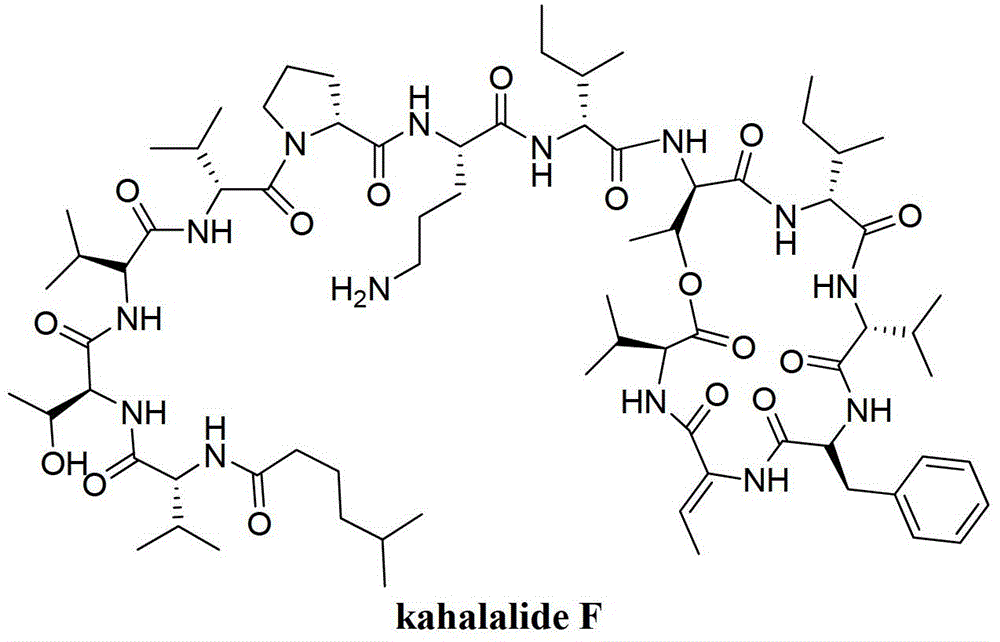
A kind of method for preparing kahalalide F
A technology of resin and fragments, which is applied in the field of preparing Kahalalide F, can solve the problems of difficult purification of intermediate reactants, low yield, and difficult removal of impurities, and achieve the effects of mild reaction conditions, single product configuration, and easy post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
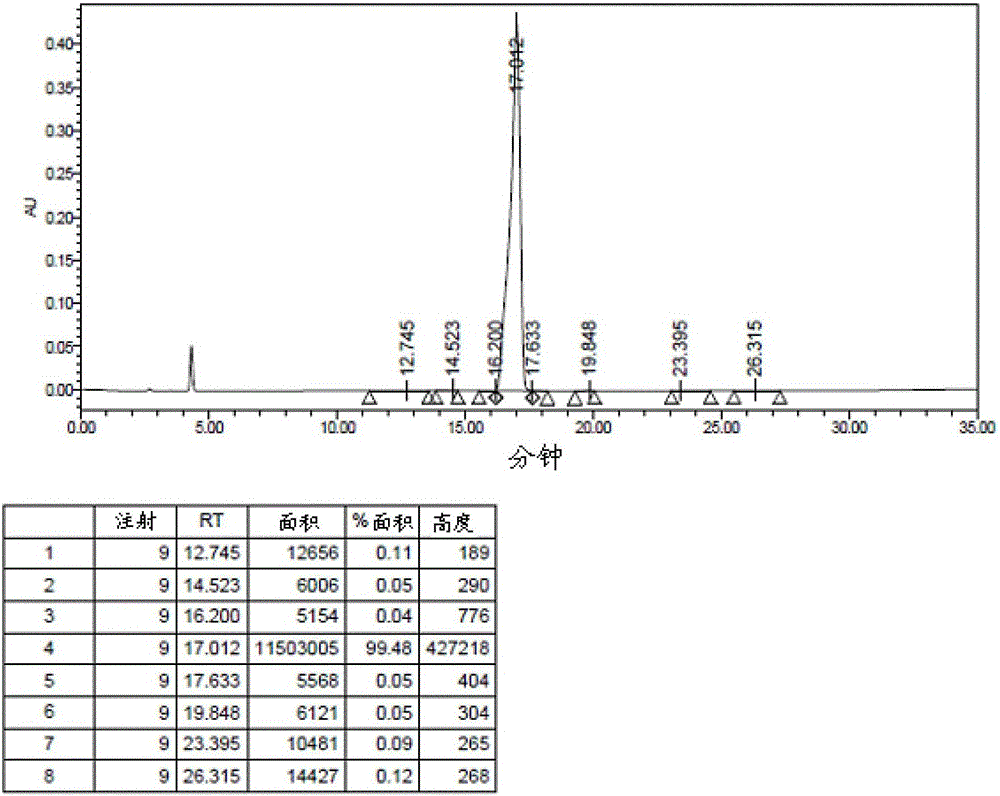
Examples
preparation example Construction
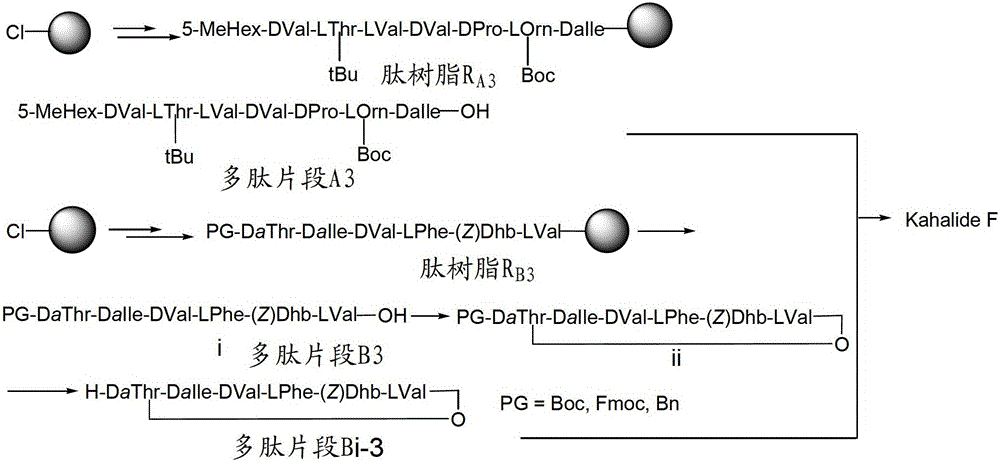
[0035] 2) Using the solid-phase peptide synthesis method to sequentially couple the corresponding amino acids to obtain the peptide resin R A and R B , the solid-phase polypeptide synthesis method comprises: i) removing Fmoc, and then washing the resin with a solvent until the complete removal of Fmoc is detected by a detection method; ii) adding an appropriate amount of amino acid to be coupled and a coupling agent in a solvent After dissolving and activating in the medium, add them together into the solid reaction column until the reaction is detected to be terminated by the detection method; iii) repeat i) and ii).
[0036] The reagent for removing Fmoc can be any reagent known in the art that can achieve this purpose, preferably 20% piperidine / DMF solution (DBLK), i.e. piperidine: DMF (volume ratio) is a mixed solution of 1:4 .
[0037] Wherein solid-phase polypeptide synthesis needs to use activator, the activator used in the present invention is the composition of DIPC...
Embodiment 1
[0069] Embodiment 1: Preparation of Fmoc-D-Pro-CTC resin
[0070] Weigh 221g of dry 2-CTC resin (the degree of substitution is 0.736mmol / g) and add it to the solid-phase reaction column. First, wash the resin with DMF for 2 times, and then use 2-3 times the volume of the resin bed to swell the resin for 1 hour. , and then washed 3 times with DMF and 2 times with DCM in turn, and kept for use.
[0071] Under the condition of cooling in an ice bath, dissolve 109.8g Fmoc-D-Pro-OH and 42.1g DIPEA in a mixed solvent of DMF and DCM. After the amino acid is dissolved, slowly dissolve the activated Fmoc-D-Pro-OH Add to the reaction column to react for 2 hours, after the reaction, add a blocking solution composed of 100mL methanol and 21.0g DIPEA to the reaction column to react for 30 minutes. After washing with DMF for 3 times, the Fmoc-D-Pro-CTC resin was obtained.
Embodiment 2
[0072] Embodiment 2: the preparation of Fmoc-L-Orn (Boc)-CTC resin
[0073] Weigh 288g of dry 2-CTC resin (the degree of substitution is 0.534mmol / g) and add it to the solid-phase reaction column. First, wash the resin with DMF twice, and then use 2-3 times the volume of the resin bed to swell the resin for 1 hour. , and then washed 3 times with DMF and 2 times with DCM in turn, and kept for use.
[0074] Under the condition of cooling in an ice bath, dissolve 139.8g Fmoc-L-Orn(Boc)-OH and 39.8g DIPEA in a mixed solvent of DMF and DCM. After the amino acid is dissolved, slowly dissolve the activated Fmoc-L-Orn (Boc)-OH was added to the reaction column to react for 2 hours. After the reaction, a blocking solution consisting of 95 mL methanol and 20.0 g DIPEA was added to the reaction column to react for 30 minutes. After washing with DMF for 3 times, the Fmoc-L-Orn(Boc)-CTC resin was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com