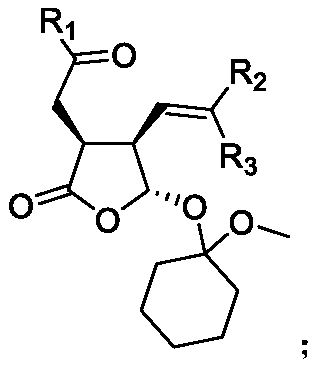
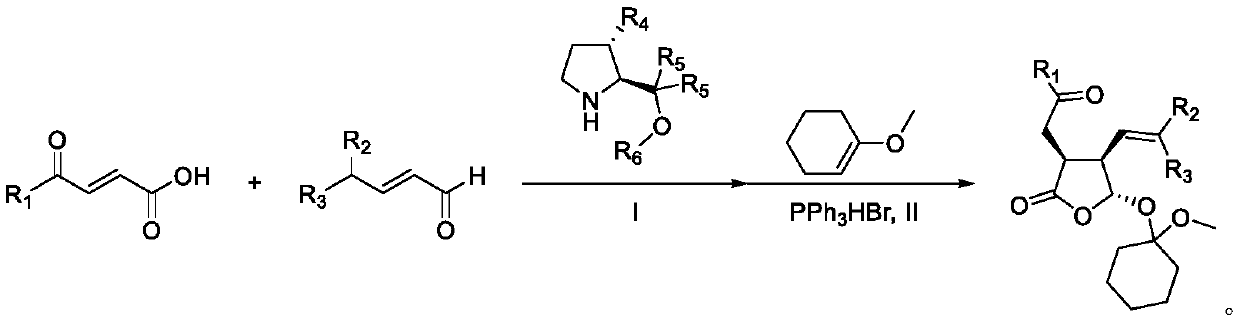
Synthetic method of multi-chiral-center gamma-lactone compound
A technology of ester compounds and chiral centers, which is applied in the field of synthesis of polychiral center γ-lactone compounds, can solve the problems of single product configuration, difficult substrate construction, and inability to obtain a single configuration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
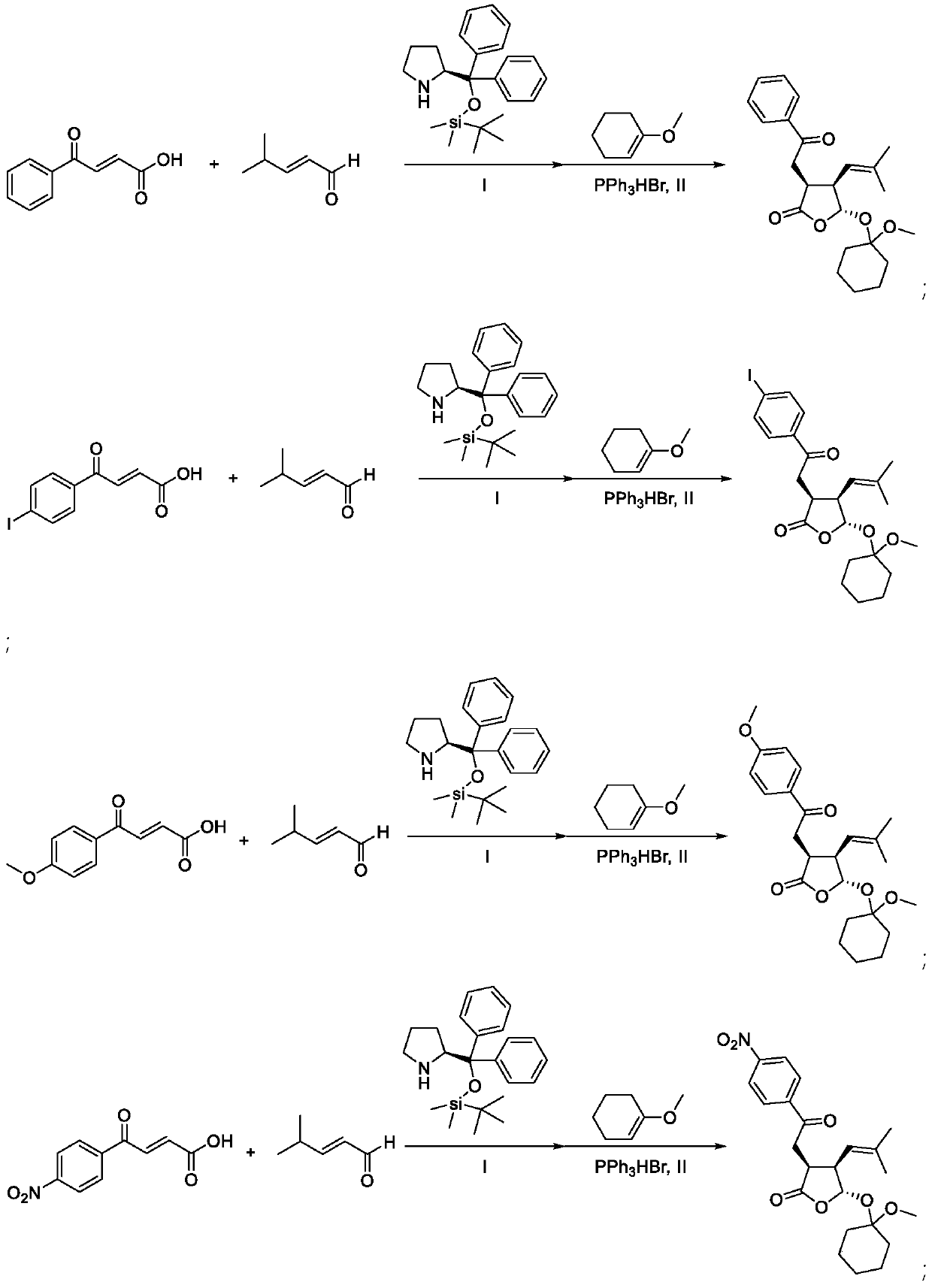
[0058] 1 mmol R 1 = 4-carbonyl-α,β-unsaturated carboxylic acid of phenyl, 2 mmol R 2 = R 3 = methylated α,β-unsaturated aldehyde, 0.2 mmol R 4 =H,R 5 = phenyl, R 6 = Diaryl prolinol silicon ether of dimethyl tert-butyl silyl, 5 ml of diethyl ether was incubated at 25°C for 48 hours; the solution obtained from the reaction was evaporated to dryness, and 0.1 mmol of triphenylphosphine hydrogen bromide was added to the reaction mixture Salt, 10 mmol 1-methoxy-1-cyclohexene, 10 ml 1,2-dichloroethane at 25 ° C for 5 h, evaporated to dryness, purified by column chromatography, the obtained colorless liquid Ⅰ 224 mg, the yield is : 58%, 99% ee
[0059] The compound of the above compound I is
[0060] HRMS: calculated for C 23 h 30 o 5 [M+H] + : 387.21660, Found 387.21657.HPLC analysis: Chiralpak IC, n-hexane / i-PrOH=90 / 10, flow rate 1.0 ml / min, λ=254nm, t major =11.8min,t minor =14.4min.
Embodiment 2
[0062] 1 mmol R 1 = 4-carbonyl-α,β-unsaturated carboxylic acid of 4-iodophenyl, 2 mmol R 2 = R 3 = methyl α,β-unsaturated aldehyde, 0.2 mmol R 4 =H,R 5 = phenyl, R 6 = Diaryl prolinol silicon ether of dimethyl tert-butyl silyl, 5 ml of diethyl ether was incubated at 25°C for 48 hours; the solution obtained from the reaction was evaporated to dryness, and 0.1 mmol of triphenylphosphine hydrogen bromide was added to the reaction mixture Salt, 10 mmoles of 1-methoxy-1-cyclohexene, 10 ml of 1,2-dichloroethane at 25°C for 5 hours, evaporated to dryness, and purified by column chromatography to obtain 388 mg of white solid II with a yield of: 76%, 99% ee
[0063] The compound of the above-mentioned compound II is
[0064] 35.53, 33.59, 25.81, 23.50, 23.38, 17.96. [α] D20 =11.33, (c 0.3, CHCl 3 ); HRMS: calculated for C 23 h 29 IO 5 [M+H] + : 513.11324, Found 513.11306.HPLC analysis: Chiralpak IC, n-hexane / i-PrOH=90 / 10, flow rate 1.0ml / min, λ=254nm, t major =12.7min,t ...
Embodiment 3
[0066] 1 mmol R 1 = 4-carbonyl-α,β-unsaturated carboxylic acid of 4-methoxyphenyl, 2 mmol R 2 = R 3 = methyl α,β-unsaturated aldehyde, 0.2 mmol R 4 =H,R 5 = phenyl, R 6 = Diaryl prolinol silicon ether of dimethyl tert-butyl silyl, 5 ml of diethyl ether was incubated at 25°C for 48 hours; the solution obtained from the reaction was evaporated to dryness, and 0.1 mmol of triphenylphosphine hydrogen bromide was added to the reaction mixture Salt, 10 mmol 1-methoxy-1-cyclohexene, 10 ml 1,2-dichloroethane at 25°C for 5 h, evaporated to dryness, purified by column chromatography, the obtained colorless liquid III 333 mg, yield For: 80%, 98% ee.
[0067] The compound of the above-mentioned compound III is
[0068] 35.90, 35.18, 33.62, 25.83, 25.82, 23.52, 23.40, 17.97. [α] D20 =-9.33, (c 0.3, CHCl 3 ); HRMS: calculated for C 24 h 32 o 6 [M+H] + : 417.22717, Found 417.22706.HPLC analysis: Chiralpak IC, n-hexane / i-PrOH=80 / 20, flow rate 1.0ml / min, λ=254nm, t major =12.6mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com