Preparation method and application of C23-38 cycloaliphatic diisocyanate
A technology of diisocyanate and cycloaliphatic diisocyanate, which is applied in the field of preparation of C23-38 cycloaliphatic diisocyanate, can solve the problems of difficult production, difficult separation, large molecular weight, etc., and achieve low production difficulty, low cost, and high configuration single effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation C 23 Cycloaliphatic diisocyanate
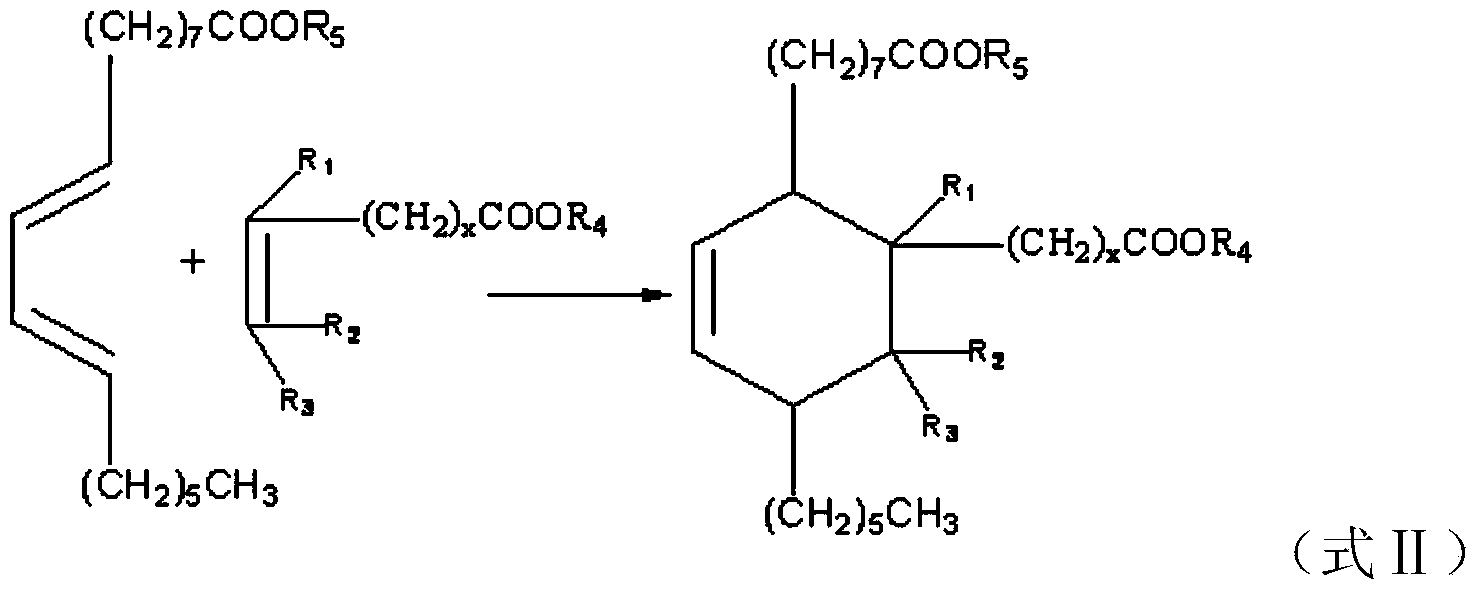
[0037] Step 1: add 500g (1.7mol) dehydrated methyl ricinoleate, 146.2g (1.7mol) methyl acrylate, and inhibitor hydroquinone 1.5g in the 2000mL pressure reactor, and replace the air in the kettle with nitrogen, Heating to 160-180°C and reacting for 3.5 hours to obtain the crude dibasic acid methyl ester.
[0038] It should be noted that the reaction of dehydrated ricinoleic acid methyl ester and methyl acrylate in this embodiment can be replaced by the reaction of dehydrated ricinoleic acid and acrylic acid, and the dibasic acid methyl ester in the following steps can also be the corresponding binary Acids, in view of space, are not given one by one, and in this embodiment, only the reaction of dehydrated ricinoleic acid methyl ester and methyl acrylate is taken as an example.
[0039] Step 2: Add 380 g (1.0 mol) of the dibasic acid methyl ester generated in step 1 and 5 g of tributyl phosphate into a 1000 mL fo...
Embodiment 2
[0053] Example 2: Preparation C 24 Cycloaliphatic diisocyanate
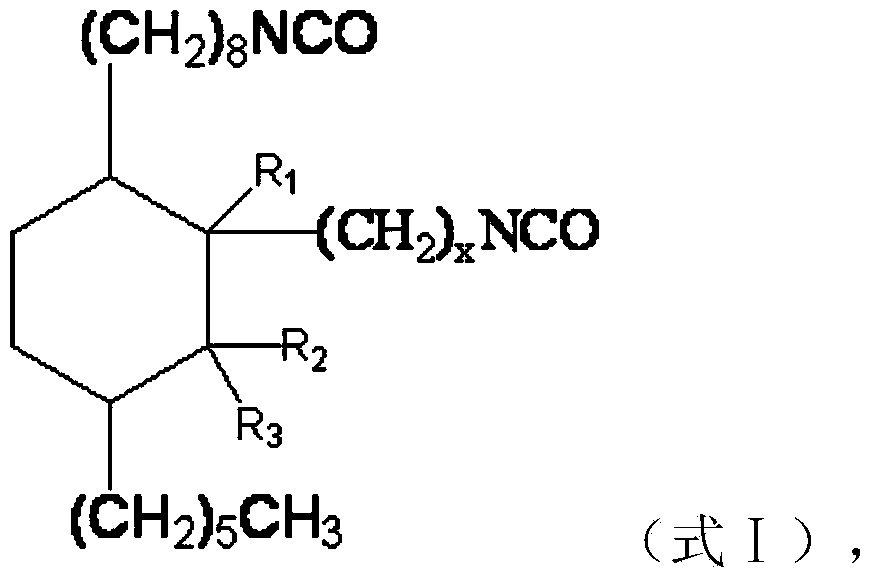
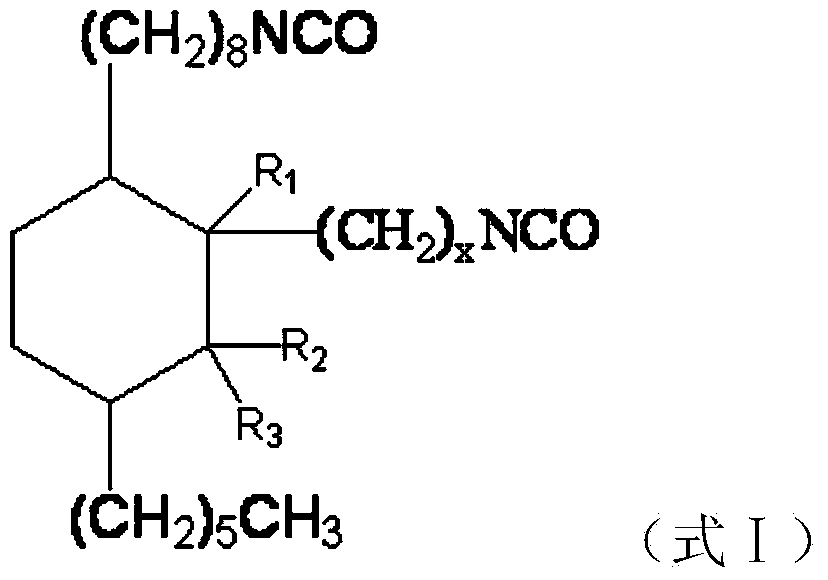
[0054] The difference between this embodiment and Example 1 is that the raw materials for preparing cycloaliphatic diisocyanate are different, and the present embodiment uses 500g (1.7mol) dehydrated methyl ricinoleate and 170.0g (1.7mol) α-methyl methacrylate Reaction, preparation process is identical with embodiment 1, obtains target product C 24 Aliphatic diisocyanate, namely 1-hexyl-3'-methyl-3-(2-isocyanatoethyl)-4-(8-isocyanatooctyl)cyclohexane.
[0055] Molecular formula: C 24 h 42 N 2 o 2
[0056] Molecular weight: 390
[0057] Appearance: light yellow oily liquid
[0058] NCO content: 19.4-21.5%
[0059] Structural formula:
[0060]
[0061] The infrared spectrometry data are as follows:
[0062] IR(kBr)γ(cm -1 ):2853.7, 2924.4 (-CH 2 ,-CH 3 C-H stretching vibration);
[0063] 1463.81354.2 (C-CH 3 , C-H deformation vibration);
[0064] 723.0–(CH 2 ) 4 skeleton vibration;
[0065] 2...
Embodiment 3
[0066] Example 3: Preparation C 24 Cycloaliphatic diisocyanate
[0067] The difference between this embodiment and Example 1 is that the raw materials for preparing cycloaliphatic diisocyanate are different, and the present embodiment uses 500g (1.7mol) dehydrated methyl ricinoleate and 170.0g (1.7mol) β-methyl methacrylate Reaction, preparation process is identical with embodiment 1, obtains target product C 24 Aliphatic diisocyanate, namely 1-hexyl-2-methyl-3-(2-isocyanatoethyl)-4-(8-isocyanatooctyl)cyclohexane.
[0068] Molecular formula: C 24 h 42 N 2 o 2
[0069] Molecular weight: 390
[0070] Appearance: light yellow oily liquid
[0071] NCO content: 19.4-21.5%
[0072] Structural formula:
[0073]
[0074] The infrared spectrometry data are as follows:
[0075] IR(kBr)γ(cm -1 ):2853.7, 2924.4 (-CH 2 ,-CH 3 C-H stretching vibration);
[0076] 1463.81354.2 (C-CH 3 , C-H deformation vibration);
[0077] 723.0–(CH 2 ) 4 skeleton vibration;
[0078] 22...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com