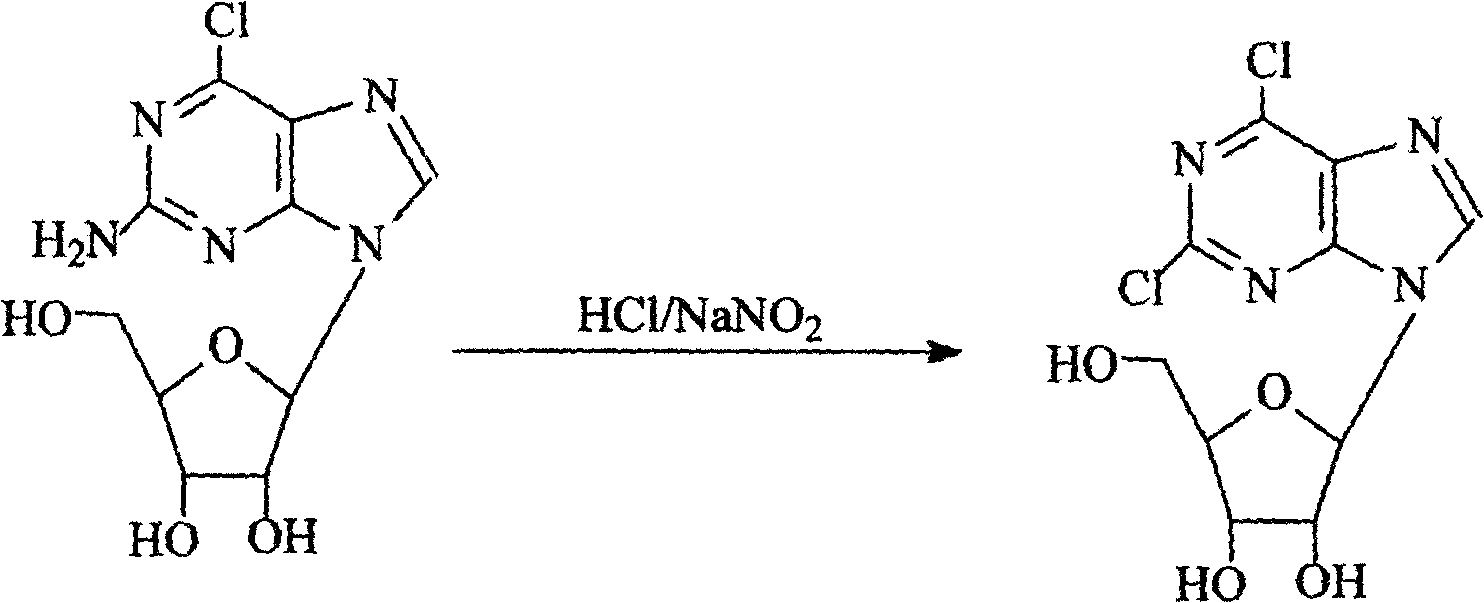
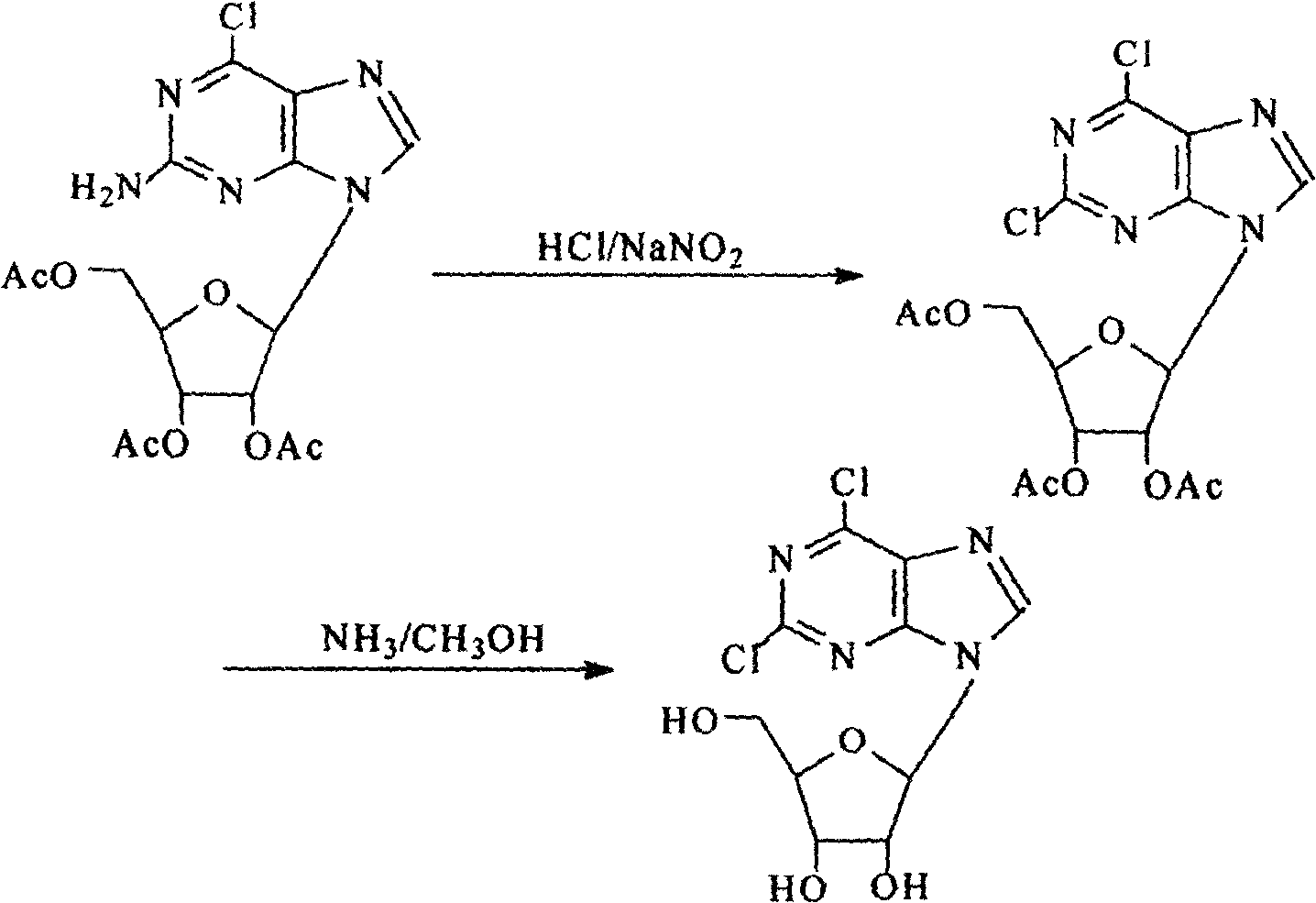
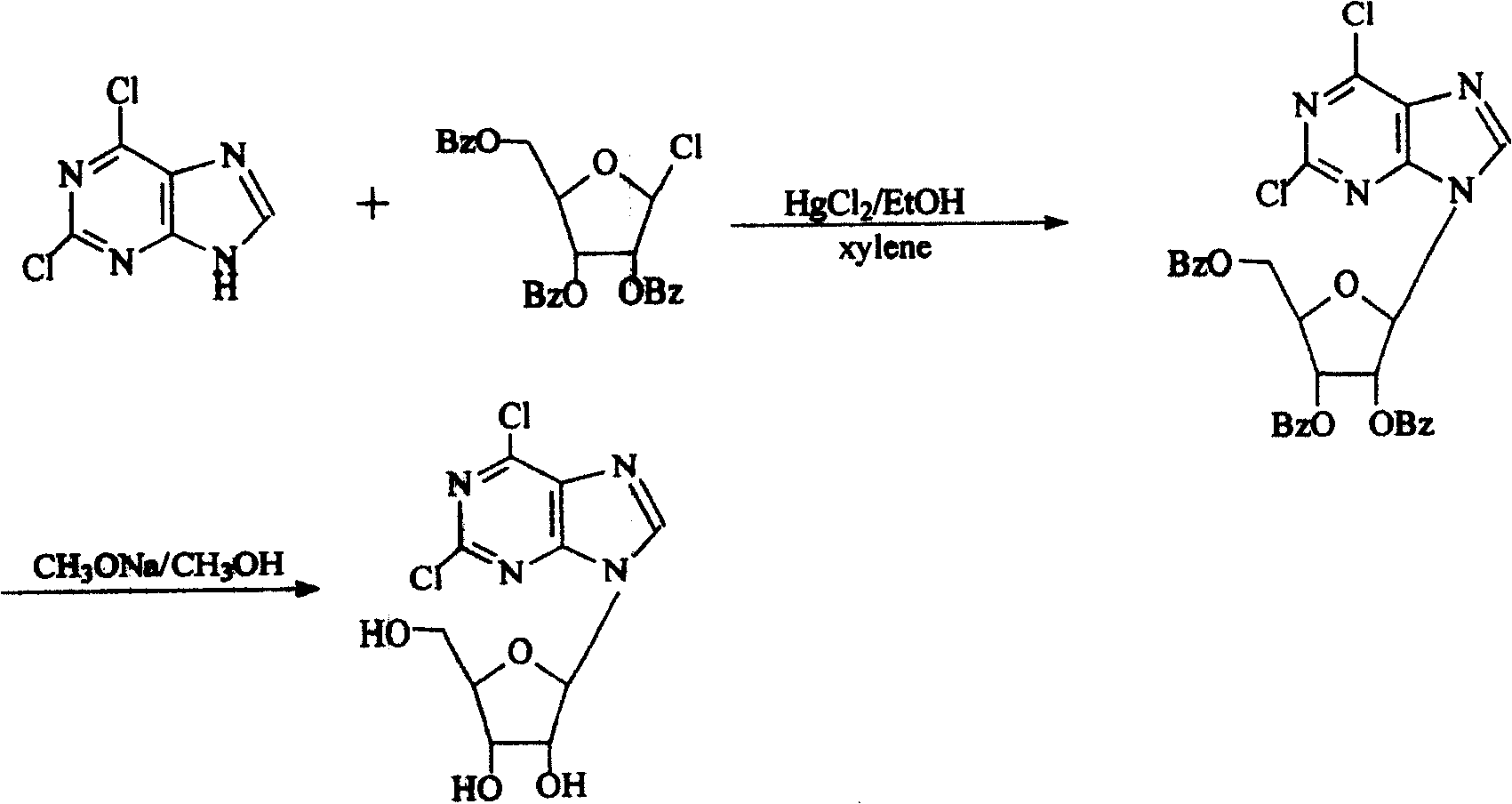
Technique for producing 2,6-dichlorine purine nucleosides by chemical synthesis method
A technology of dichloropurine nucleoside and dichloropurine nucleoside high-quality products, applied in the field of chemical synthesis production 2, can solve the problems of difficult separation, reduced yield, non-single aminolysis products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 500Kg, β-D-1,2,3,5-tetraacetyl ribofuranose (II) into a 10,000-liter stainless steel reactor with stirring and heat to melt, then add 189Kg in batches under stirring 2,6 -dichloropurine (I) and 1.6kg of bis-p-nitrophenol phosphate, heated to reflux under stirring, connected to a vacuum to remove the acetic acid generated by the reaction in time, after the reaction was completed, remove the vacuum to obtain 2,6-dichloro-9 -(β-D-2',3',5'-triacetyl ribofuranose)purine (III) 371-402Kg, the conversion rate is 83-90%.
[0028] (2) Add 371Kg (III) and 2200 liters of methanol into a 10,000-liter stainless steel reactor with stirring, add concentrated hydrochloric acid 4 dropwise at 0°C-5°C, keep the temperature and continue the reaction until the reaction is complete (1-4h ), continue to keep the temperature at 0°C-5°C, add solid NaHCO3 to adjust to pH=7. Suction filtration under reduced pressure, and the filtrate was concentrated to dryness under reduced pressure to o...
Embodiment 2
[0030] (1) Add 321Kg, β-D-1,2,3,5-tetraacetyl ribofuranose (II) and 600 liters of dichloroethane in a 10,000-liter stainless steel reactor with stirring, and add in batches under stirring 189Kg2, 6-dichloropurine (I) and 1.6kg bis-p-nitrophenol phosphonate are heated to reflux under stirring, after the completion of the reaction, connect the vacuum to remove the acetic acid generated by the solvent and the reaction, and after vacuum drying, get 2 , 6-dichloro-9-(β-D-2′,3′,5′-triacetyl ribofuranose)purine (III) 304-344Kg, the conversion rate is 68-77%.
[0031] (2) Add 304Kg (III) and 2000 liters of methanol into a 10,000-liter stainless steel reactor with stirring, add concentrated hydrochloric acid 4 dropwise at 0°C-5°C, keep the temperature and continue the reaction until the reaction is complete (1-4h ), continue to keep the temperature at 0°C-5°C, add solid NaHCO3 to adjust to pH=7. Suction filtration under reduced pressure, and the filtrate was concentrated to dryness un...
Embodiment 3
[0033] (1) Add 500Kg, β-D-1,2,3,5-tetraacetyl ribofuranose (II) into a 10,000-liter stainless steel reactor with stirring and heat to melt, then add 189Kg2,6- Dichloropurine (I) and 1.8kg of two p-fluorophenol phosphates were heated to reflux under stirring, and the acetic acid generated by the reaction was removed in time by vacuuming, and after the reaction was completed, the vacuum was removed to obtain 2,6-dichloro-9- (β-D-2', 3', 5'-triacetyl ribofuranose) purine (III) 286-344Kg, the conversion rate is 64-77%.
[0034] (2) Add 286Kg (III) and 1900 liters of methanol into a 10,000 liter stainless steel reactor with stirring, add concentrated hydrochloric acid 4 dropwise at 0°C-5°C, keep the temperature and continue the reaction until the reaction is complete (1-4h ), continue to keep the temperature at 0°C-5°C, add solid NaHCO3 to adjust to pH=7. Suction filtration under reduced pressure, and the filtrate was concentrated to dryness under reduced pressure to obtain off-wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com