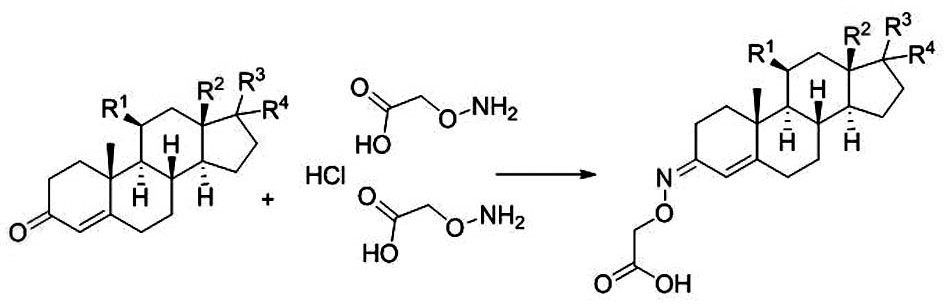
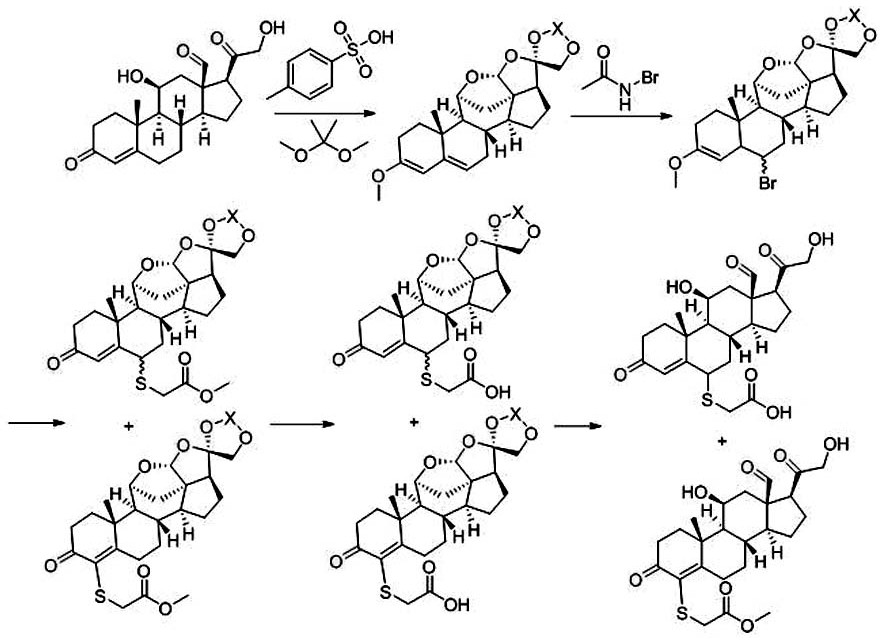
A kind of synthetic method of steroid derivative containing carboxyl group in a kind of 4-position
A synthetic method and derivative technology, applied in organic chemistry, steroids, etc., can solve the problems of unsatisfactory antibody adaptability and specific binding efficiency, long synthetic route, long reaction time, etc., and achieve single configuration, The effect of short synthesis cycle and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
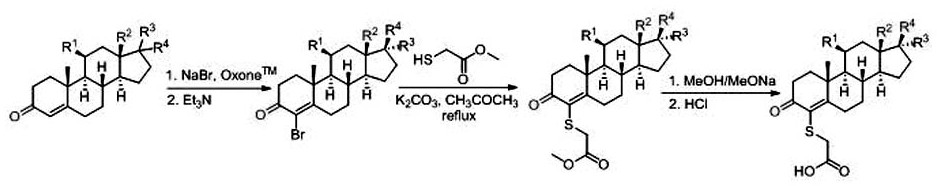
[0022] The 4-position of the present invention contains the carboxyl steroid derivative synthetic method comprising the following specific steps:
[0023] In the first step, the steroid hormone is dissolved in an organic solvent (dimethyl sulfoxide, tetrahydrofuran, methanol or ethanol, etc.) and then reacted with potassium peroxymonosulfonate and sodium bromide aqueous solution. During the reaction, the steroid hormone and peroxide Potassium oxymonosulfonate and sodium bromide first form an epoxy intermediate and then undergo a ring-opening reaction; the reaction temperature is 20~30°C, and the reaction time is 2~3h; in order to improve the conversion rate of steroid hormones, steroid hormones and The molar ratio of potassium peroxymonosulfonate and sodium bromide can be 1:1.1~1.5:1.2~1.6;
[0024] In the second step, the product (4-brominated steroid derivative) obtained in the first step is coupled with methyl thioglycolate under potassium carbonate as the base and reflux c...
Embodiment 1
[0031] Example 1 Synthesis of aldosterone derivatives at the 4-position carboxyl group
[0032]Add aldosterone (11β,21-dihydroxy-3.20-dioxo-4-pregnene-18-al (11→18) lactaldehyde) into a 50mL Schlenk tube, then add 10mL of tetrahydrofuran, after the aldosterone dissolves, Potassium peroxymonosulfonate (1.2eq.), sodium bromide aqueous solution (1.4eq.) and triethylamine (2.0eq.) were added sequentially according to the molar ratio, and the reaction was stirred at room temperature for 3 hours. Spot the plate to monitor the completion of the reaction, concentrate the organic solvent, add water to quench the reaction, extract and separate the liquid with ethyl acetate, mix the sample with silica gel, and purify by column chromatography (the eluent is dichloromethane: ethyl acetate = 3:1) Obtain 4-bromo aldosterone, yield 83%;
[0033] Add 4-bromoaldosterone into a 50 mL Schlenk tube, add 10 mL of acetone, add methyl thioglycolate (1.2 eq.) and potassium carbonate (1.5 eq.), and st...
Embodiment 2
[0038] Example 2 Synthesis of cortisol derivatives at the 4-position carboxyl group
[0039] Add cortisol (hydrocortisone) into a 50mL Schlenk tube, and then add 10mL of tetrahydrofuran. After the cortisol is dissolved, add potassium peroxymonosulfonate (1.2eq.), sodium bromide aqueous solution (1.3 eq.) and triethylamine (2.0eq.), stirred at room temperature for 3 hours. Spot the plate to monitor the completion of the reaction, concentrate the organic solvent, add water to quench the reaction, extract and separate the liquid with ethyl acetate, mix the sample with silica gel, and purify by column chromatography (dichloromethane: ethyl acetate = 4:1 as the eluent) Obtain 4-bromocortisol, yield 89%;
[0040] Add 4-bromocortisol into a 50 mL Schlenk tube, add 10 mL of acetone, add methyl thioglycolate (1.2 eq.) and potassium carbonate (1.5 eq.), and stir at 80°C for 8 hours under reflux. Spot the plate to monitor the completion of the reaction, concentrate the organic solvent,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com