A kind of preparation method of florfenicol intermediate flumethone oxazole
A technology for the production of florfenicol and florfenicol is applied in the field of preparation of florfenicol intermediates, which can solve the problems of sulfuryl fluoride toxicity, irritation, unfavorable environmental protection, and unsatisfactory effects, and achieve The effect of considerable yield, simple recycling and clear prospects for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
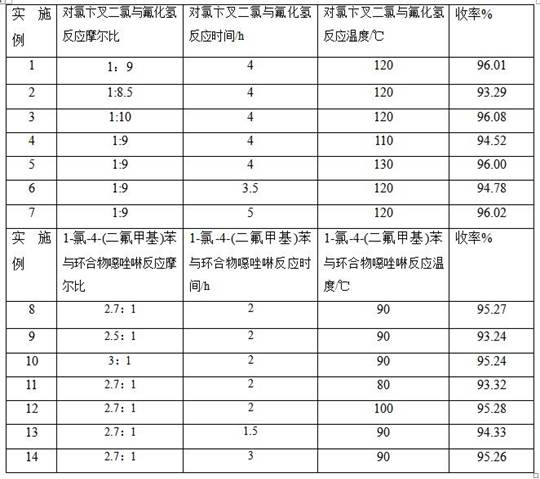
[0039] Example 1: Preparation of 1-chloro-4-(difluoromethyl)benzene
[0040] Mix 97.75 g (0.5 mol) of p-chlorobenylidene dichloride and 0.7 g (0.025 mol) of antimony trichloride in an autoclave, remove the air in the autoclave under reduced pressure, suck in 90 g (4.5 mol) of hydrogen fluoride gas at room temperature, and heat up React at 120°C for 4 hours, cool to 40°C, discharge and collect the gas in the kettle, blow it clean with nitrogen (200g, 7.14mol), cool HF to liquid by cryogenic cooling, absorb the remaining gas with water, and prepare a hydrochloric acid solution. The product 1-chloro-4-(difluoromethyl)benzene was distilled off under reduced pressure from the remaining mixture in the kettle, and the fraction at 60°C-65°C / 2.4Kpa was collected, cooled and liquefied to obtain the product as a colorless liquid (78g, 0.480mol). The yield is 96.01% (calculated on the basis of p-chloro-benylidene dichloride), and the chromatographic purity is 99.80% (higher than the purit...
Embodiment 2
[0041]Embodiment two: the preparation of 1-chloro-4-(difluoromethyl)benzene
[0042] Mix 97.77g (0.5mol) of p-chlorobenylidene dichloride and 0.7g (0.025mol) of antimony trichloride in the autoclave, remove the air in the autoclave under reduced pressure, suck in 85g (4.25mol) of hydrogen fluoride gas at room temperature, and heat up React at 120°C for 4 hours, cool to 40°C, discharge and collect the gas in the kettle, blow it clean with nitrogen (200g, 7.14mol), cool HF to liquid by cryogenic cooling, absorb the remaining gas with water, and prepare a hydrochloric acid solution. The product 1-chloro-4-(difluoromethyl)benzene was distilled off under reduced pressure from the remaining mixture in the kettle, and the fraction at 60°C-65°C / 2.4Kpa was collected, cooled and liquefied to obtain the product as a colorless liquid (75.8g, 0.466mol) , Yield 93.29% (calculated on the basis of p-chloro-Benylidene dichloride), chromatographic purity 99.81%.
Embodiment 3
[0043] Example three: Preparation of 1-chloro-4-(difluoromethyl)benzene
[0044] Mix 97.75 g (0.5 mol) of p-chlorobenylidene dichloride and 0.7 g (0.025 mol) of antimony trichloride in the autoclave, remove the air in the autoclave under reduced pressure, suck in 100 g (5.0 mol) of hydrogen fluoride gas at room temperature, and heat up React at 120°C for 4 hours, cool to 40°C, discharge and collect the gas in the kettle, blow it clean with nitrogen (200g, 7.14mol), cool HF to liquid by cryogenic cooling, absorb the remaining gas with water, and prepare a hydrochloric acid solution. The product 1-chloro-4-(difluoromethyl)benzene was distilled off under reduced pressure from the remaining mixture in the kettle, and the fraction at 60°C-65°C / 2.4Kpa was collected, cooled and liquefied to obtain the product as a colorless liquid (78.05g, 0.481mol) , Yield 96.08% (calculated on the basis of p-chloro-Benylidene dichloride), chromatographic purity 99.80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com