Reaction accelerator, urethane compound using same, thiourethane compound, and production method for amide compound or urea compound
A technology of reaction accelerators and compounds, which is applied in the field of production of reaction accelerators and carbamate compounds, thiocarbamate compounds, amide compounds or urea compounds using them, can solve the physical properties of cured products Adverse effects, insufficient promotion effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0151] Hereinafter, the present invention will be described in detail by way of examples, but the present invention is not limited by these examples.
[0152] In each of the following examples, unless otherwise specified, "%" means mass % (wt%), and "ppm" means mass ppm (wtppm).
[0153] The conditions of liquid chromatography analysis (hereinafter referred to as "LC analysis") are as follows.
[0154] Columns: Showa Denko Co., Ltd. product name "Shodex (registered trademark) KF-801" 4 pieces,
[0155] Eluent: tetrahydrofuran (THF),
[0156] Flow rate: 0.8mL / min,
[0157] Oven temperature: 40°C,
[0158] Detector: differential refractive index (RI) UV (wavelength 210nm)
[0159] (Preparation method of reaction accelerator)
Synthetic example 1
[0161] Into a 100 mL three-necked flask, 10.0 g of methacryloyloxyethyl isocyanate (Karenz MOI (registered trademark), manufactured by Showa Denko Co., Ltd., hereinafter referred to as "MOI") was put, and the inner temperature was cooled to 15° C. while 2.58 g of dry hydrogen chloride was bubbled into methacryloyloxyethyl isocyanate through an inner tube to obtain 12.6 g of methacryloyloxyethylcarbamoyl chloride (hereinafter referred to as "MOC"). The purity is 100%.
Synthetic example 2
[0163] 110 g of aminoethyl methacrylate hydrochloride (hereinafter referred to as "AEMHCl") was put into 200 g of toluene, and 110 g of phosgene was supplied while AEMHCl was melted at an internal temperature of 85°C to synthesize MOI. Dissolved phosgene was removed by bubbling nitrogen gas into the reaction solution, and toluene as a solvent was further distilled off under reduced pressure to obtain 110 g of crude MOI.
[0164] The MOC content in the crude MOI was confirmed by silver nitrate titration and found to be 10.8%.
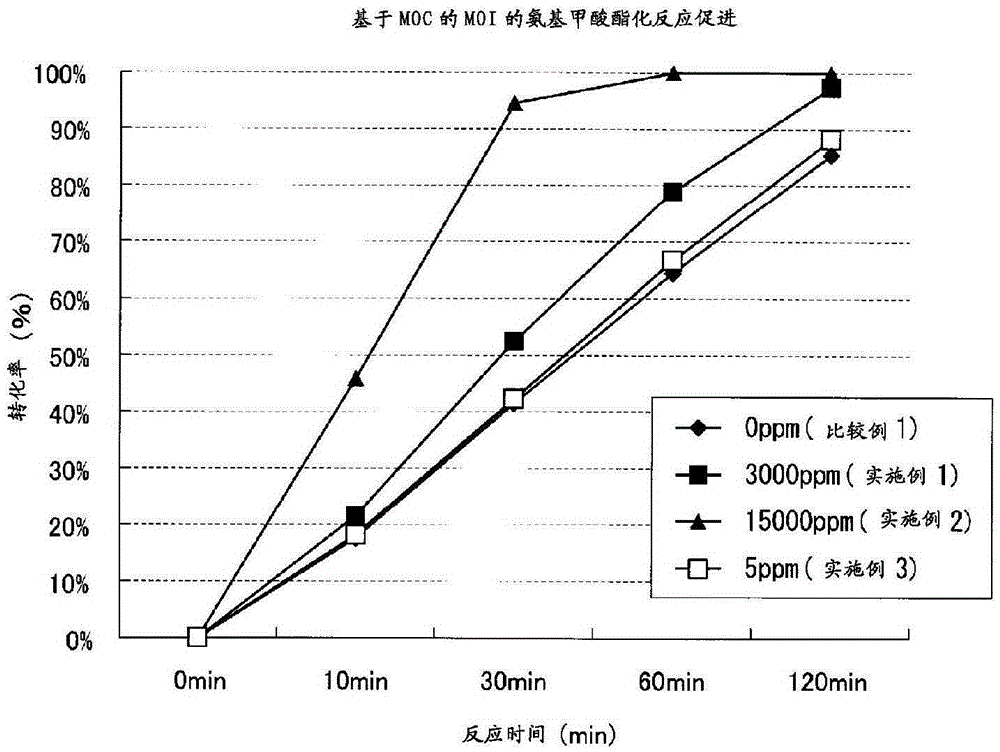
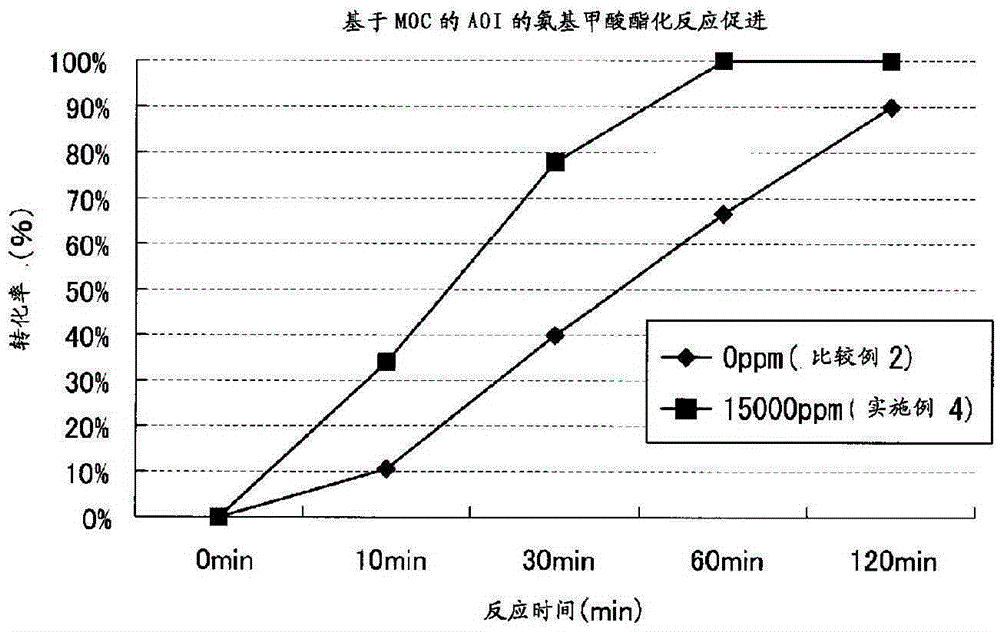
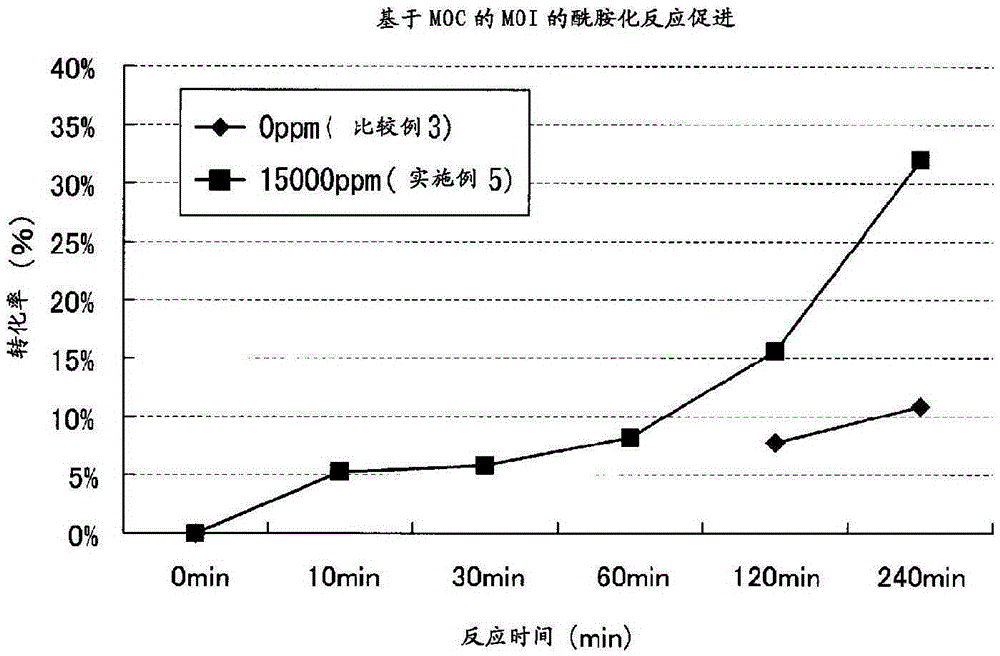
[0165] (Acceleration effect of urethanization reaction by addition of reaction accelerator)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com