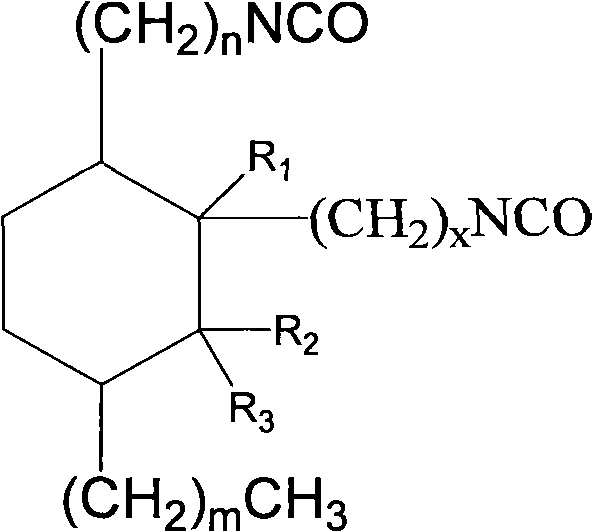
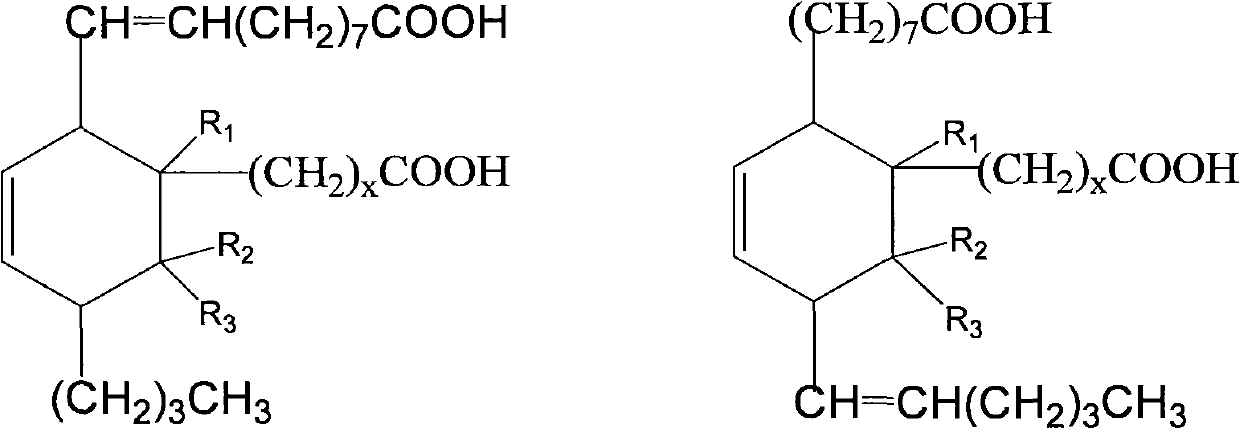
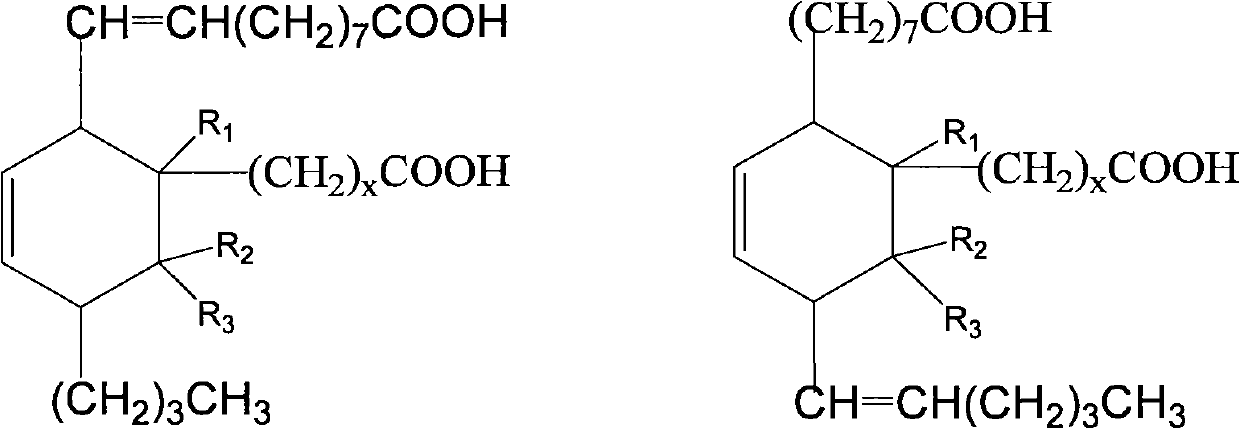
Aliphatic diisocyanate and preparation method and purposes thereof
A diisocyanate and aliphatic technology, applied in the field of C21-36 aliphatic diisocyanate and its preparation, can solve the problems of harsh process conditions, easy occurrence of side reactions, and difficulty in industrialization, avoiding side reactions, being beneficial to industrial promotion and application, Less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Preparation of C21 aliphatic diisocyanate
[0062] step 1:
[0063] Add 500g (1.7mol) methyl oleate, 123.5g (1.7mol) methyl acrylate and 1.5g hydroquinone to a 2000mL pressure reactor, replace the air in the kettle with nitrogen, heat up to 160-180°C, and react After 3.5 hours, the crude dibasic acid ester was obtained. The resulting product is a mixture of the following dibasic esters, and its structural formula is:
[0064]
[0065] Cool down and pump the reaction mixture into another 2 liter autoclave, then add 500g methanol and 30gPd (5%) / activated carbon catalyst (wet: 15g dry weight, 0.75gPd), vacuumize, nitrogen replacement, hydrogen replacement respectively. The reaction mixture was stirred at 160-170° C., 1.5-2.0 Mp for about 7.5 hours, until the hydrogen absorption was complete, and the stirring was stopped. The material was filtered off under nitrogen pressure, leaving the catalyst in the filter. With 500g methanol, the catalyst is backwa...
Embodiment 2
[0078] Example 2: C 22 Aliphatic diisocyanate
[0079] step 1:
[0080] Add 500g (1.7mol) methyl oleate, 171.7g (1.7mol) α-methyl methacrylate and 1.5g hydroquinone to a 2000mL pressure reactor, replace the air in the kettle with nitrogen, and heat up to 120~ 140°C, reacted for 3.5 hours to obtain crude dibasic acid. The resulting product is a mixture of the following dibasic esters, and its structural formula is:
[0081]
[0082] Cool down and pump the reaction mixture into another 2-liter autoclave, then add 500g ethanol and 30gPt (5%) / activated carbon catalyst (wet: dry weight 15g, 0.75gPd), vacuumize, nitrogen replacement, hydrogen replacement respectively. The reaction mixture was stirred at 150-180° C., 2.0-2.5 Mp for about 8 hours, until the hydrogen absorption was complete, and the stirring was stopped. The material was filtered off under nitrogen pressure, leaving the catalyst in the filter. With 500g of ethanol, the catalyst is backwashed into the reactor, a...
Embodiment 3
[0095] Example 3: C 22 Aliphatic diisocyanate
[0096] step 1:
[0097] Add 500g (1.7mol) methyl oleate, 171.7g (1.7mol) β-methyl methacrylate (methyl crotonate) and 1.5g hydroquinone to a 2000mL pressure reactor, and replace the air in the kettle with nitrogen , heated to 160-180°C, and reacted for 3.5 hours to obtain crude dibasic acid. The resulting product is a mixture of the following dibasic esters, and its structural formula is:
[0098]
[0099] Cool down and pump the reaction mixture into another 2 liter autoclave, then add 500g methanol and 30gPd (5%) / activated carbon catalyst (wet: 15g dry weight, 0.75gPd), vacuumize, nitrogen replacement, hydrogen replacement respectively. The reaction mixture was stirred at 160-170° C., 1.5-2.0 Mp for about 7.5 hours, until the hydrogen absorption was complete, and the stirring was stopped. The material was filtered off under nitrogen pressure, leaving the catalyst in the filter. With 500g methanol, the catalyst is backwas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com