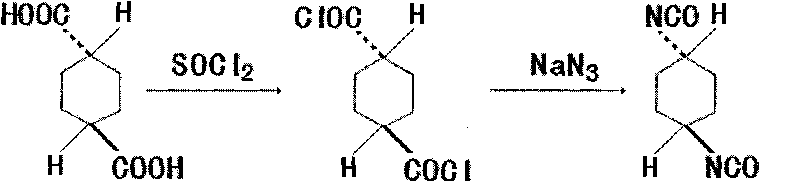
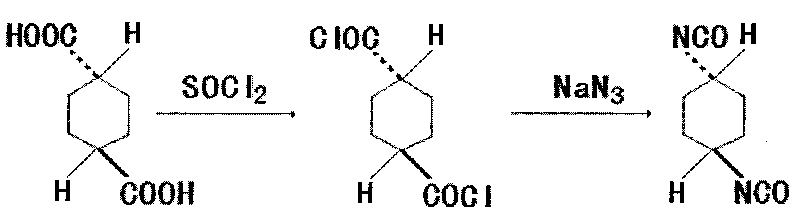
Method for synthesizing trans-1,4-cyclohexane diisocyanate
A technology of cyclohexane diisocyanate and cyclohexane dicarboxylic acid, which is applied in the preparation of carboxylic acid nitrogen-containing derivatives, organic chemistry and other directions, can solve the problems of short reaction period and the like, and achieves short reaction period, easy availability of raw materials, and process route. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 172.18g trans-1,4-cyclohexanedicarboxylic acid (1mol), 440ml (6mol) thionyl chloride, add in the three-necked flask of reflux condenser, stirring, thermometer and tail gas absorption device, heat and reflux for 4 hours, The excess thionyl chloride was recovered under reduced pressure to obtain 199.5 g of crude trans-1,4-cyclohexanedicarbonyl chloride, with a yield of 94.99%.
Embodiment 2
[0020] 172.18g trans-1,4-cyclohexanedicarboxylic acid (1mol), 440ml (6mol) thionyl chloride, add in the three-necked flask of reflux condenser, stirring, thermometer and tail gas absorption device, heat and reflux for 3.5 hours, The excess thionyl chloride was recovered under reduced pressure to obtain 196.9 g of crude trans-1,4-cyclohexanedicarbonyl chloride, with a yield of 94.21%.
Embodiment 3
[0022] 172.18g trans-1,4-cyclohexanedicarboxylic acid (1mol), 440ml (6mol) thionyl chloride, add in the three-necked flask of reflux condenser, stirring, thermometer and tail gas absorption device, heat and reflux for 3 hours, The excess thionyl chloride was recovered under reduced pressure to obtain 186.7 g of crude trans-1,4-cyclohexanedicarbonyl chloride, with a yield of 89.33%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com