Method for preparing formamide compound by catalyzing carbon dioxide hydrogenation with porous material
A technology of amine compounds and formamides, which is applied in the field of preparation of formamide compounds, can solve the problems of complex synthesis, unfavorable DMF industrial production, and low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Synthesis of Porous Organometallic Polymer Material 1a
[0068]
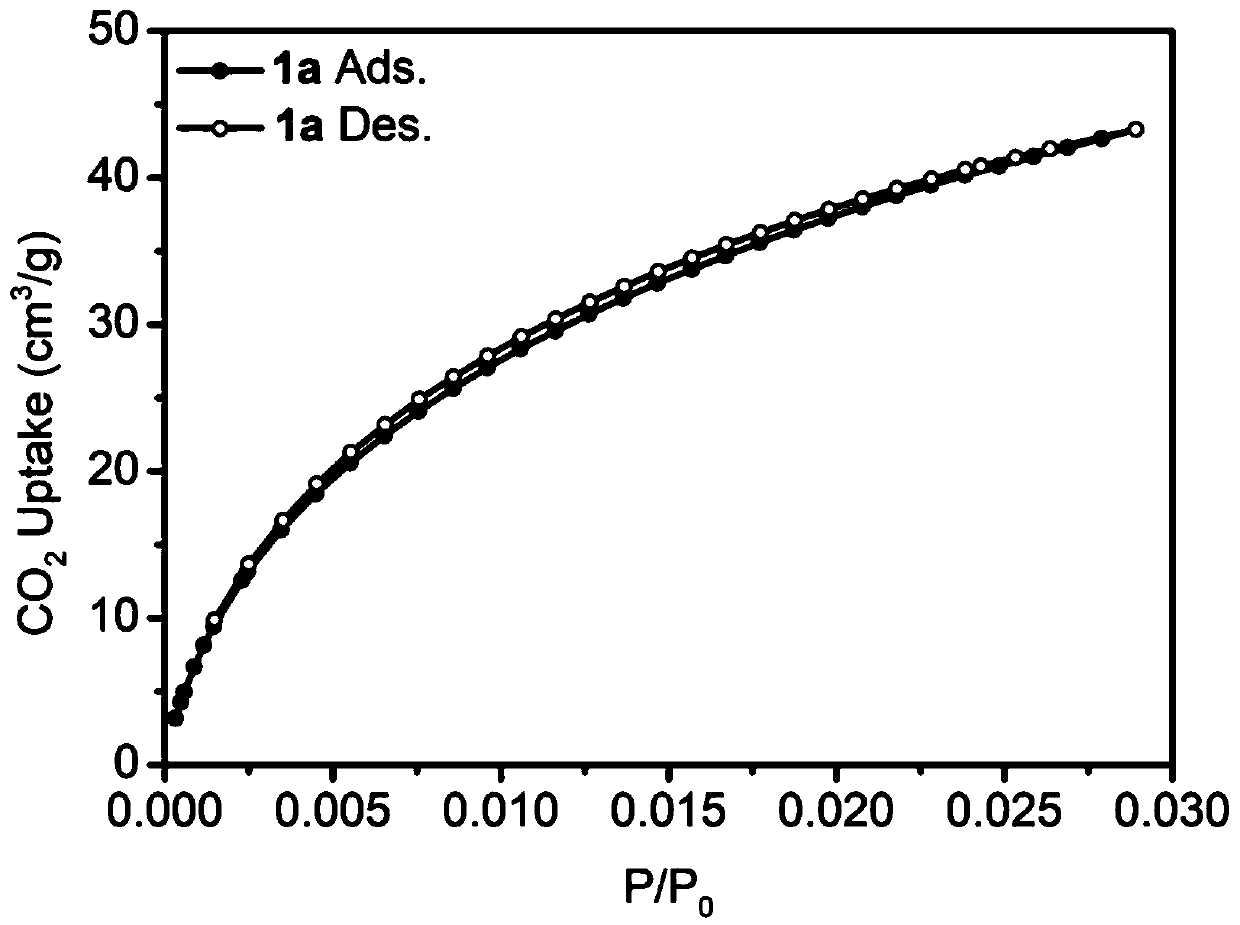
[0069] Add 1mmol of bisbenzimidazole nitrogen heterocyclic carbene iridium compound (0.63g) into a 50mL Schlenk tube, vacuum and change nitrogen three times, then add 10mL of 1,2-dichloroethane and 3mmol of benzene (0.23g) successively, and stir at room temperature After a period of time until the solids were completely dissolved, another 20 mmol of dimethylformal (FDA, 1.52 g) and anhydrous ferric chloride (3.24 g) were added. After sealing, the reaction system was placed in an oil bath at 80°C for 24 hours. After the reaction was complete, it was cooled to room temperature, filtered, washed, and the obtained solid was extracted by Soxhlet for 24 hours, and vacuum-dried at 60° C. for 24 hours to obtain the porous organometallic polymer POMP 1d. The solid carbon dioxide adsorption curve is attached figure 1 shown. Yield: 0.99 g, 90%.
Embodiment 2
[0070] Example 2: Synthesis of Porous Organometallic Polymer Material 1b
[0071]
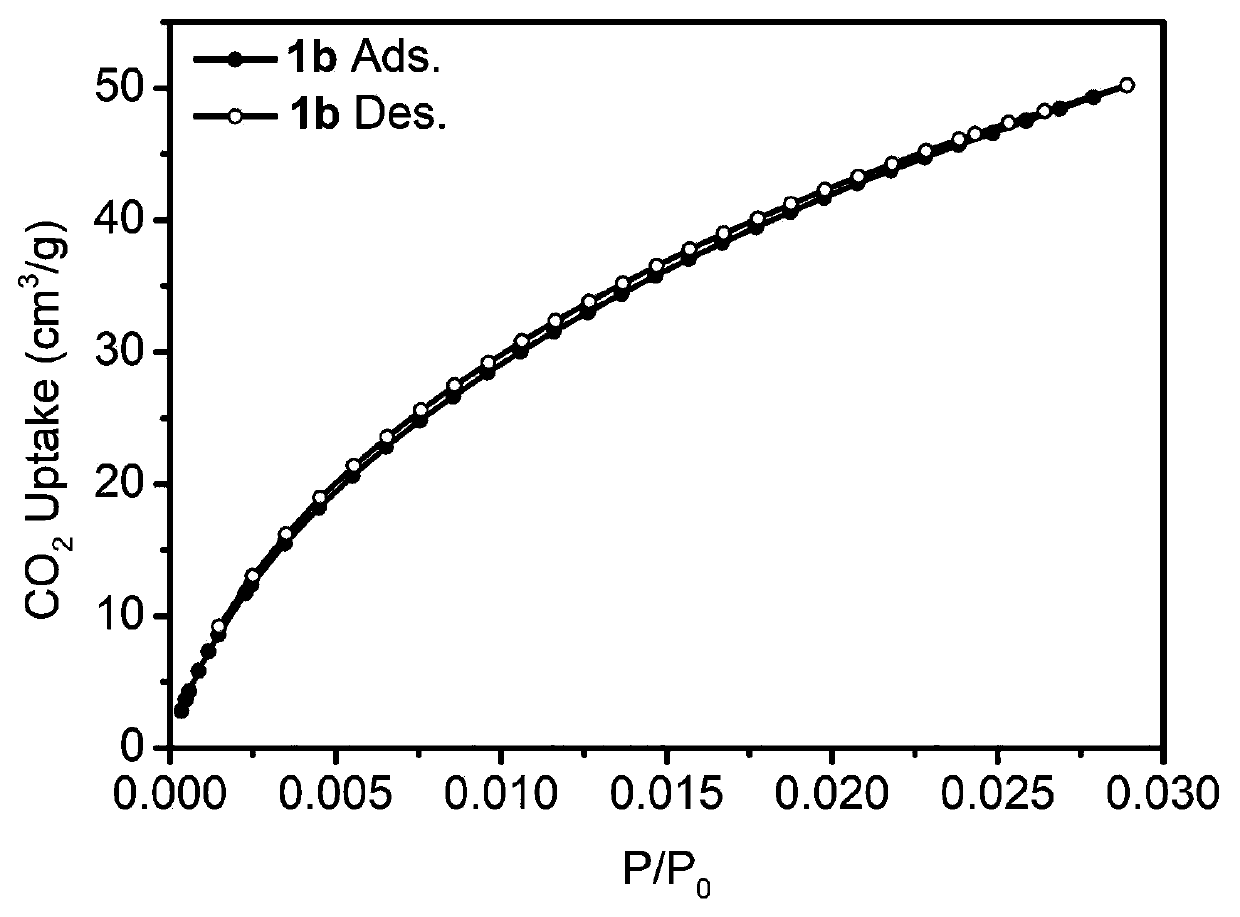
[0072] Add 1mmol of bisbenzimidazole nitrogen heterocyclic carbene iridium compound (0.63g) into a 50mL Schlenk tube, vacuum three times for nitrogen, then add 10mL of 1,2-dichloroethane and 6mmol of benzene (0.46g) successively, and stir at room temperature After a period of time until the solids were completely dissolved, another 20 mmol of dimethylformal (FDA, 1.52 g) and anhydrous ferric chloride (3.24 g) were added. After sealing, the reaction system was placed in an oil bath at 80°C for 24 hours. After the reaction was complete, it was cooled to room temperature, filtered, washed, and the obtained solid was extracted by Soxhlet for 24 hours, and vacuum-dried at 60° C. for 24 hours to obtain the porous organometallic polymer POMP 1b. The solid carbon dioxide adsorption curve is attached figure 2 shown. Yield: 1.17 g, 88%.
Embodiment 3
[0073] Example 3: Synthesis of Porous Organometallic Polymer Material 1c
[0074]
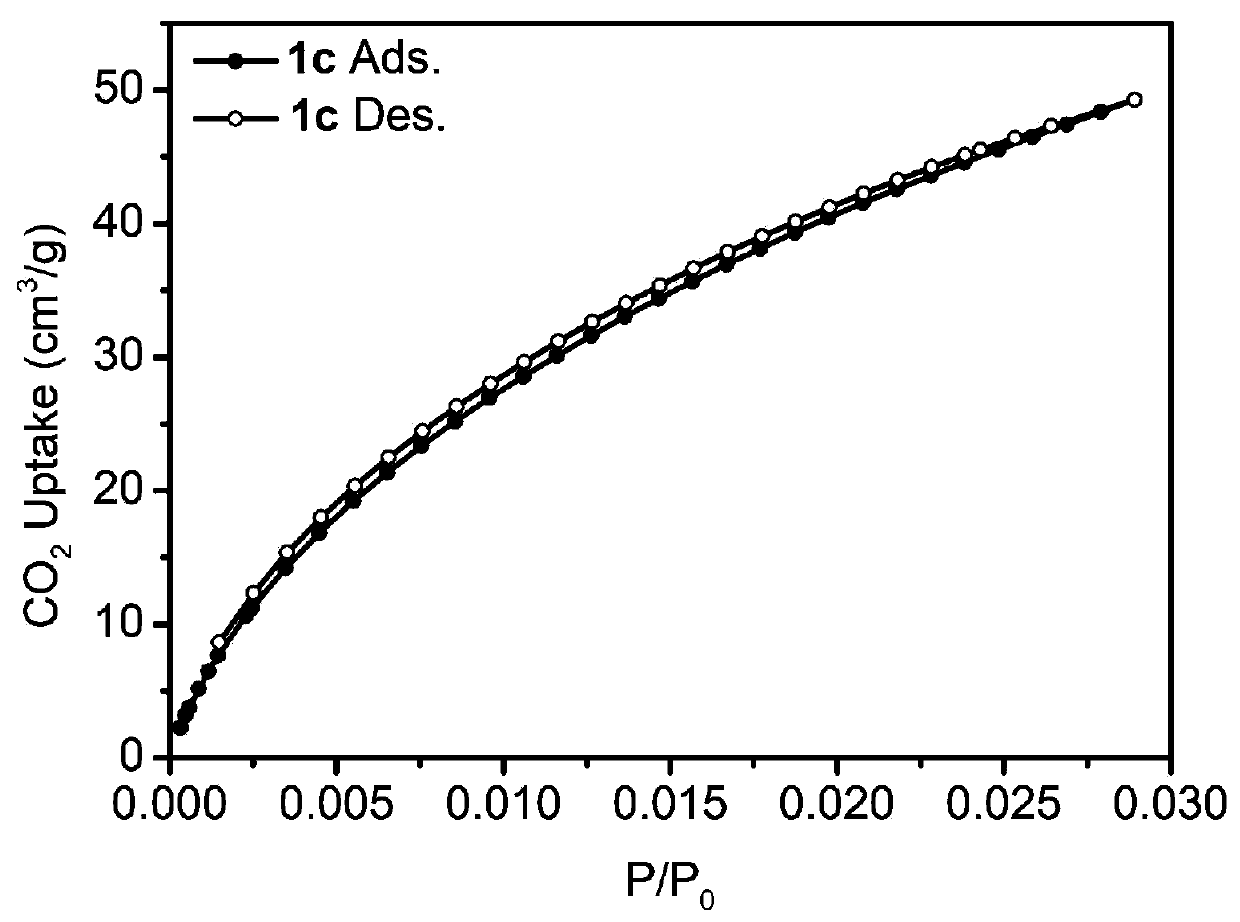
[0075] Add 1mmol of bisbenzimidazole nitrogen heterocyclic carbene iridium compound (0.63g) into a 50mL Schlenk tube, vacuum and change nitrogen three times, then add 10mL of 1,2-dichloroethane and 9mmol of benzene (0.70g) successively, and stir at room temperature After a period of time until the solids were completely dissolved, another 20 mmol of dimethylformal (FDA, 1.52 g) and anhydrous ferric chloride (3.24 g) were added. After sealing, the reaction system was placed in an oil bath at 80°C for 24 hours. After the reaction was complete, it was cooled to room temperature, filtered, washed, and the obtained solid was extracted by Soxhlet for 24 hours, and vacuum-dried at 60° C. for 24 hours to obtain the porous organometallic polymer POMP 1c. The solid carbon dioxide adsorption curve is attached image 3 shown. Yield: 1.37 g, 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com