Synthesis method for TB derivatives with human liver cancer HepG2 cell resisting activity
A synthesis method and cell activity technology, applied in the fields of drug combination, organic chemistry, anti-tumor drugs, etc., can solve the problems of easy clinical metastasis and recurrence, poor overall effect, and insignificant early symptoms of liver cancer, and achieve high practical application value, The effect of short reaction time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
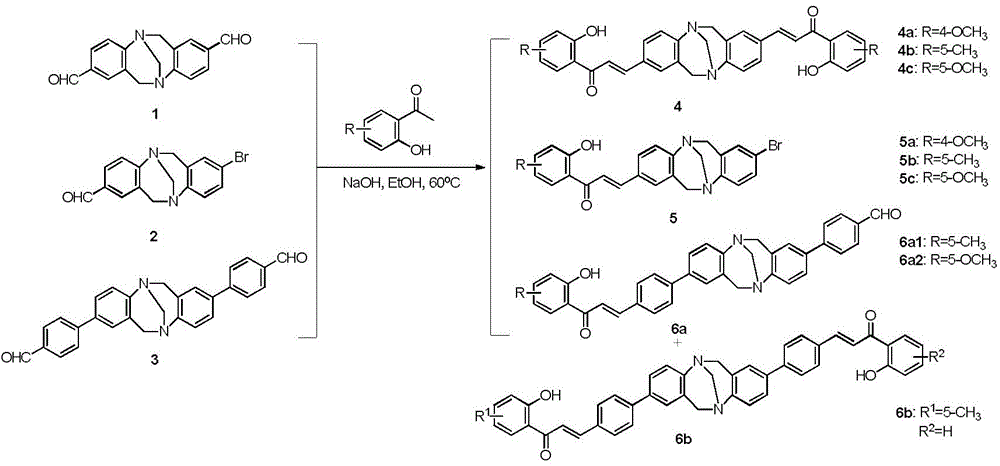
[0036] The synthesis method of the TB derivatives comprises: TB-chalcone compounds, TB-flavonoids and TB-benzimidazole compounds, and the anti-human liver cancer HepG2 cell activity of the TB derivatives is determined by the MTT method;
[0037] 1, the synthetic method of TB-chalcones compound:
[0038]
[0039] 1) Synthesis of compound 4:
[0040] Add 0.5mmol of compound 1, namely 2,8-diformyl TB, and 0.75mmol of substituted-2-hydroxyacetophenone into the round bottom flask, and the substituted-2-hydroxyacetophenone is 4-OCH 3 -2-Hydroxyacetophenone; 5-CH 3- 2-Hydroxyacetophenone or 5-OCH 3 -2-Hydroxyacetophenone, 1.8mmol NaOH and 5mL absolute ethanol, reacted at 60°C, tracked by TLC, the solution turned orange; after the reaction was completed, cooled to room temperature, added ice water, and adjusted the pH to 3-4; Suction filtration, washing with water, purification of the filter cake by column chromatography, petroleum ether: ethyl acetate = 2:1, to obtain orange po...
Embodiment 1
[0072] Embodiment 1: Take the synthesis of the target product 4a as an example.
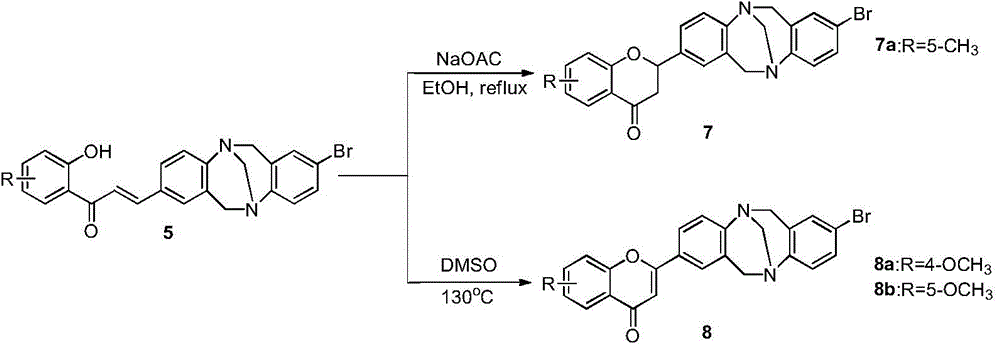
[0073] The synthesis steps include: 1) using 4-bromoaniline as the starting material to react with paraformaldehyde at low temperature to obtain the compound 2,8-dibromo-TB; 2) using 2,8-dibromo-TB as the raw material Compound 1 was obtained by formylation and Suzuki C-C coupling; 3) Compound 1 was subjected to aldol condensation reaction with 2-hydroxy-5-methylbenzophenone to obtain the target compound 4a, a total of three steps. 1) 4-bromoaniline reacts with paraformaldehyde at -15°C to generate compound 2,8-dibromo-TB, and its reaction formula is as follows:
[0074]
[0075] At -15°C, add 60mmol p-bromoaniline and 120mmol paraformaldehyde into a 250mL dry three-necked round-bottom flask, stir, and slowly drop 120mL trifluoroacetic acid into it with a constant pressure funnel to form a maroon solution. After the dropwise addition, Stirring was continued for 6 days at 0°C. After the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com