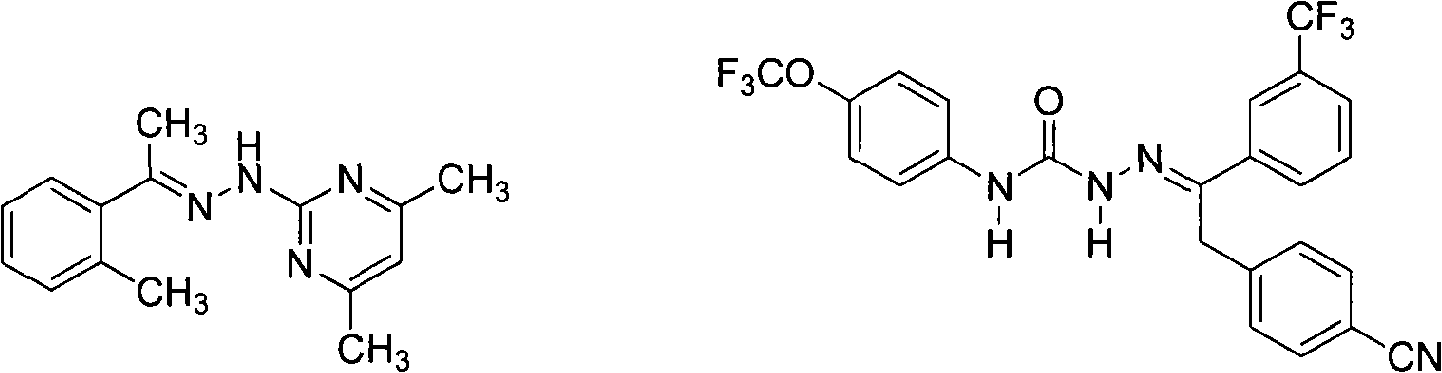
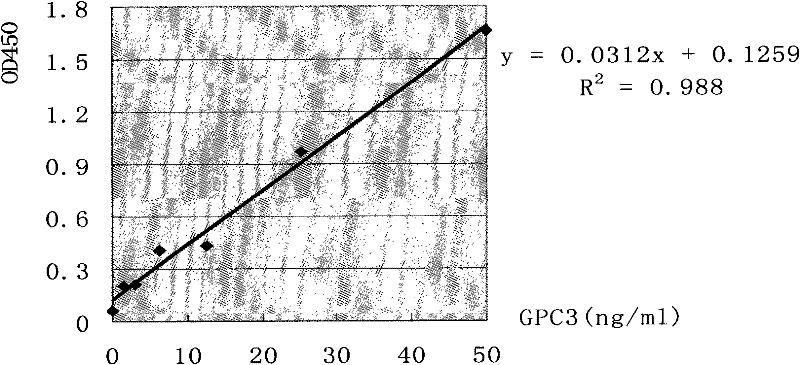
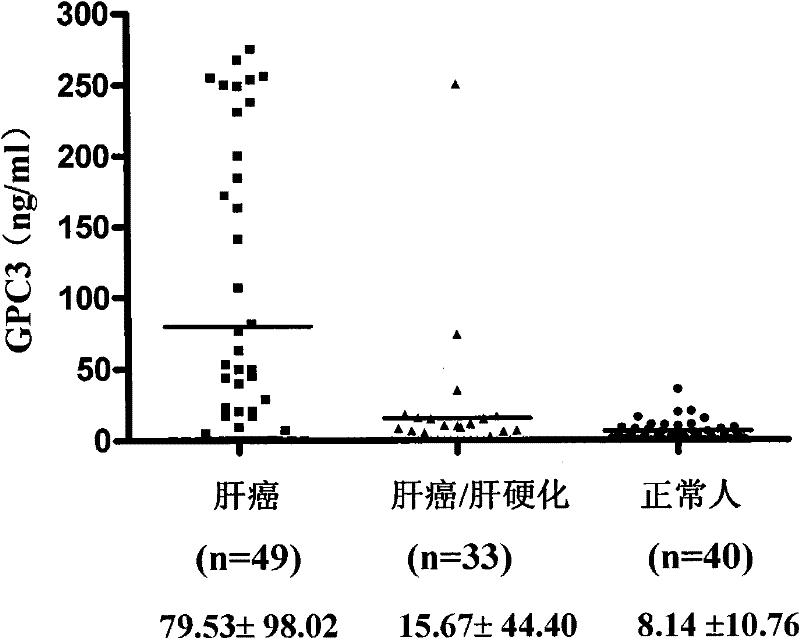
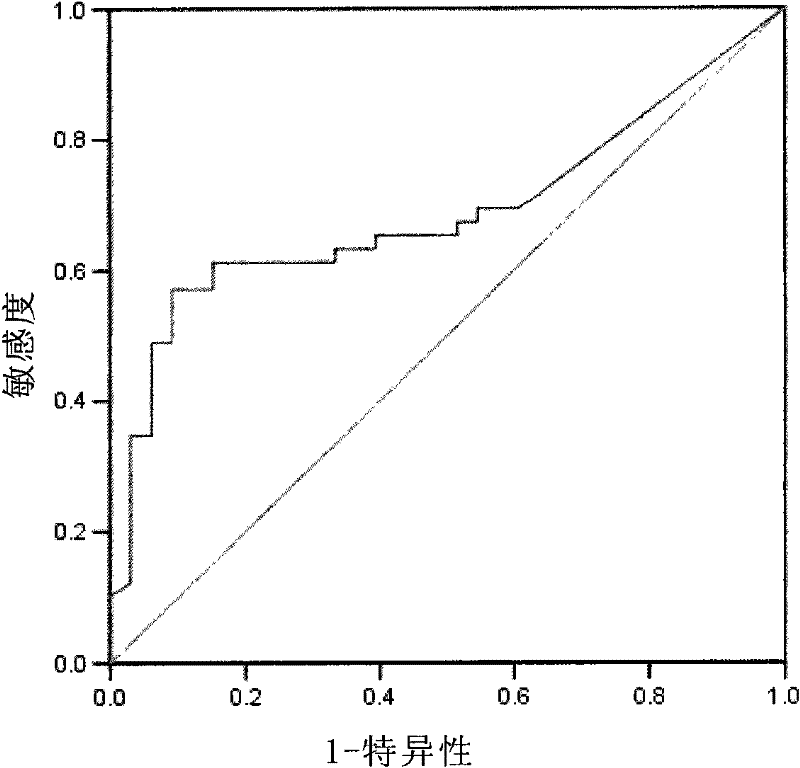
Patents
Literature
662 results about "Human liver cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liver cancer is a type of cancer that starts in the liver. Some cancers develop outside the liver and spread to the area. However, only cancers that start in the liver are described as liver cancer. The liver, which is located below the right lung and under the ribcage, is one of the largest organs of the human body.
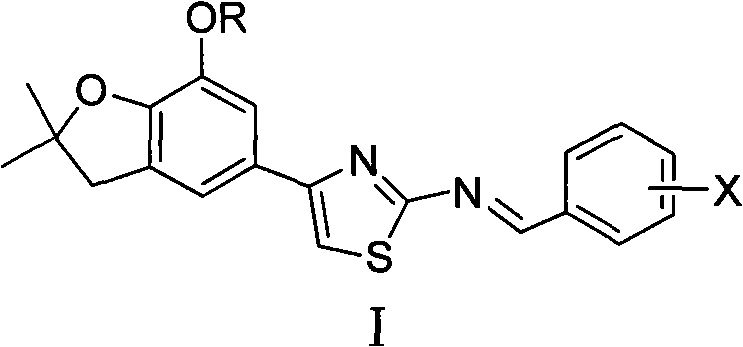
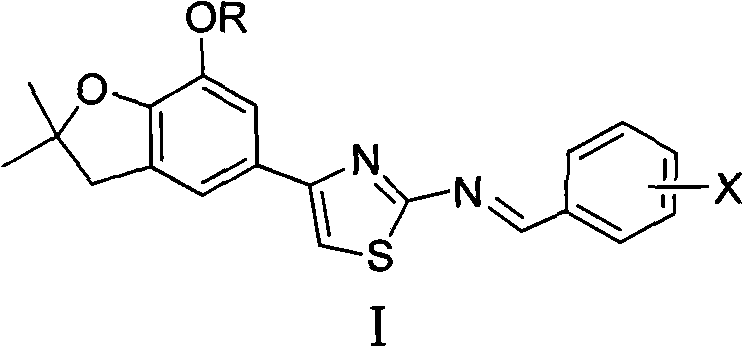
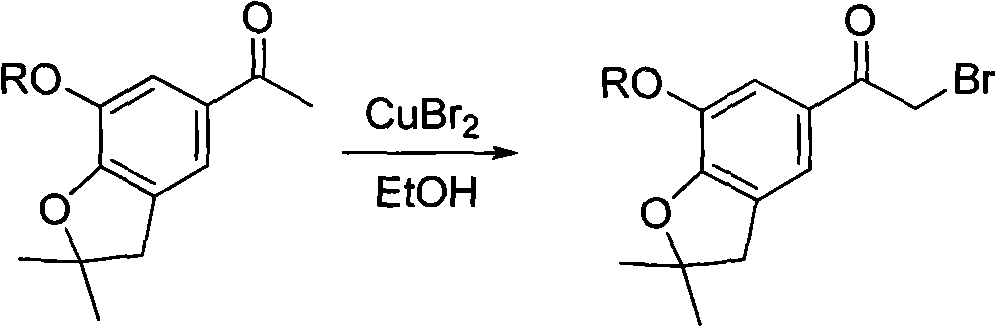
4-(benzofuran-5-yl)-2-benzal aminothiazole and application of 4-(benzofuran-5-base)-2-benzal aminothiazole as antineoplastic agent
The invention discloses 4-(benzofuran-5-yl)-2-benzal aminothiazole as shown in a chemical structural formula I. The preparation method of the 4-(benzofuran-5-yl)-2-benzal aminothiazole is as follows: 1-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl) butanone is subject to bromination and reacts with thiourea to obtain 4-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-2-aminothiazole which reacts with aromatic aldehyde to prepare the 4-(benzofuran-5-yl)-2-benzal aminothiazole. The 4-(benzofuran-5-yl)-2-benzal aminothiazole has good activity inhabiting activity on Hela cells, human liver cancer cells (Bel 7402 cells) and lung carcinoma cells (A549 cells) and can be used for preparing the antineoplastic agent.
Owner:HUNAN UNIV
Methods and compositions for the identification, assessment, prevention and therapy of human cancers
InactiveUS20020051978A1Sure easyReduced growth rateMicrobiological testing/measurementDisease diagnosisHuman cancerCancer cell
The present invention is directed to the identification of markers that can be used to determine the sensitivity of cancer cells to a therapeutic agent. The present invention is also directed to the identification of therapeutic targets. Nucleic acid arrays were used to determine the level of expression of sequences (genes) found in 60 different solid tumor cancer cell lines selected from the NCI 60 cancer cell line series. Expression analysis was used to identify markers associated with sensitivity to certain chemotherapeutic agents.
Owner:MILLENNIUM PHARMA INC
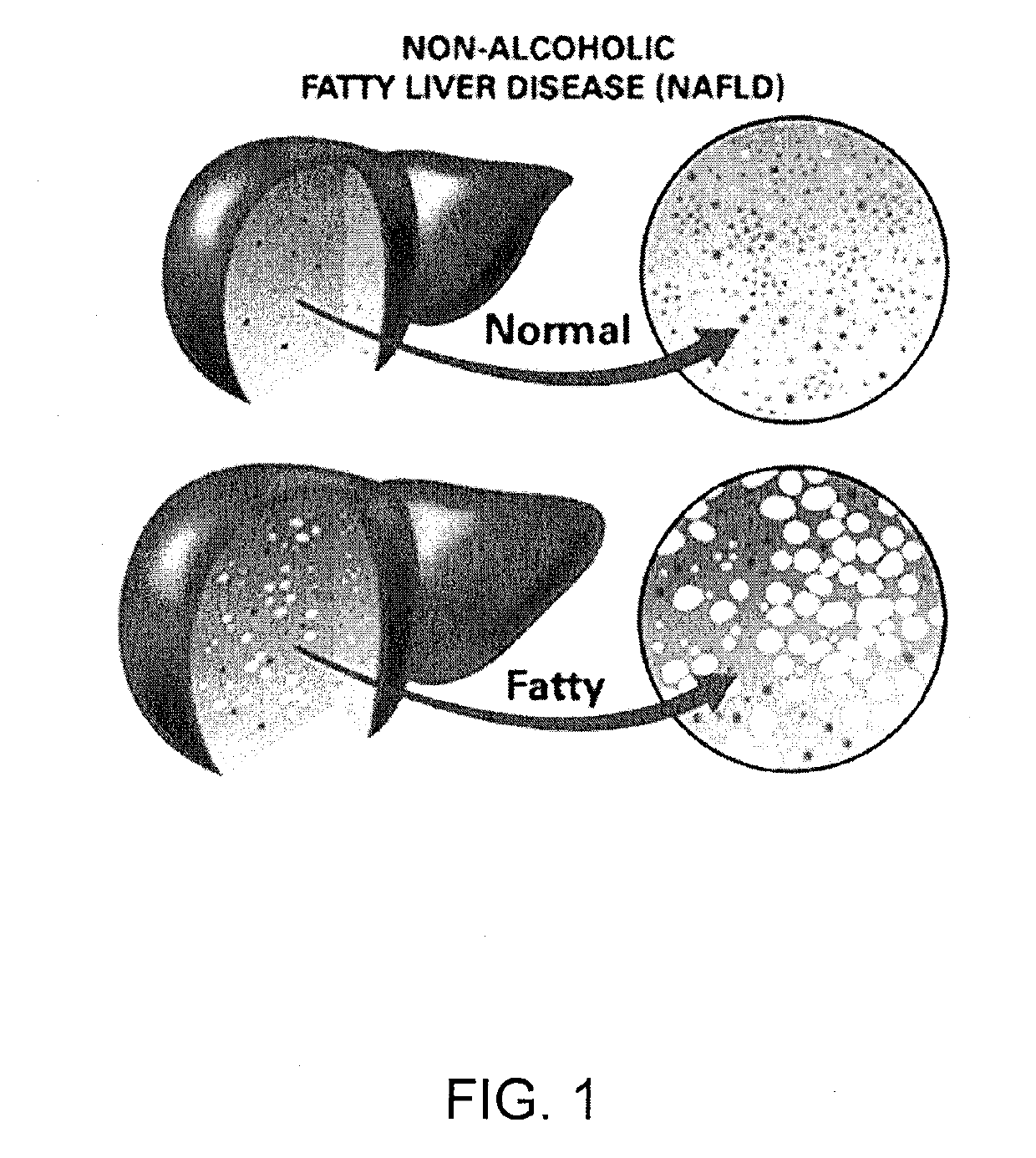
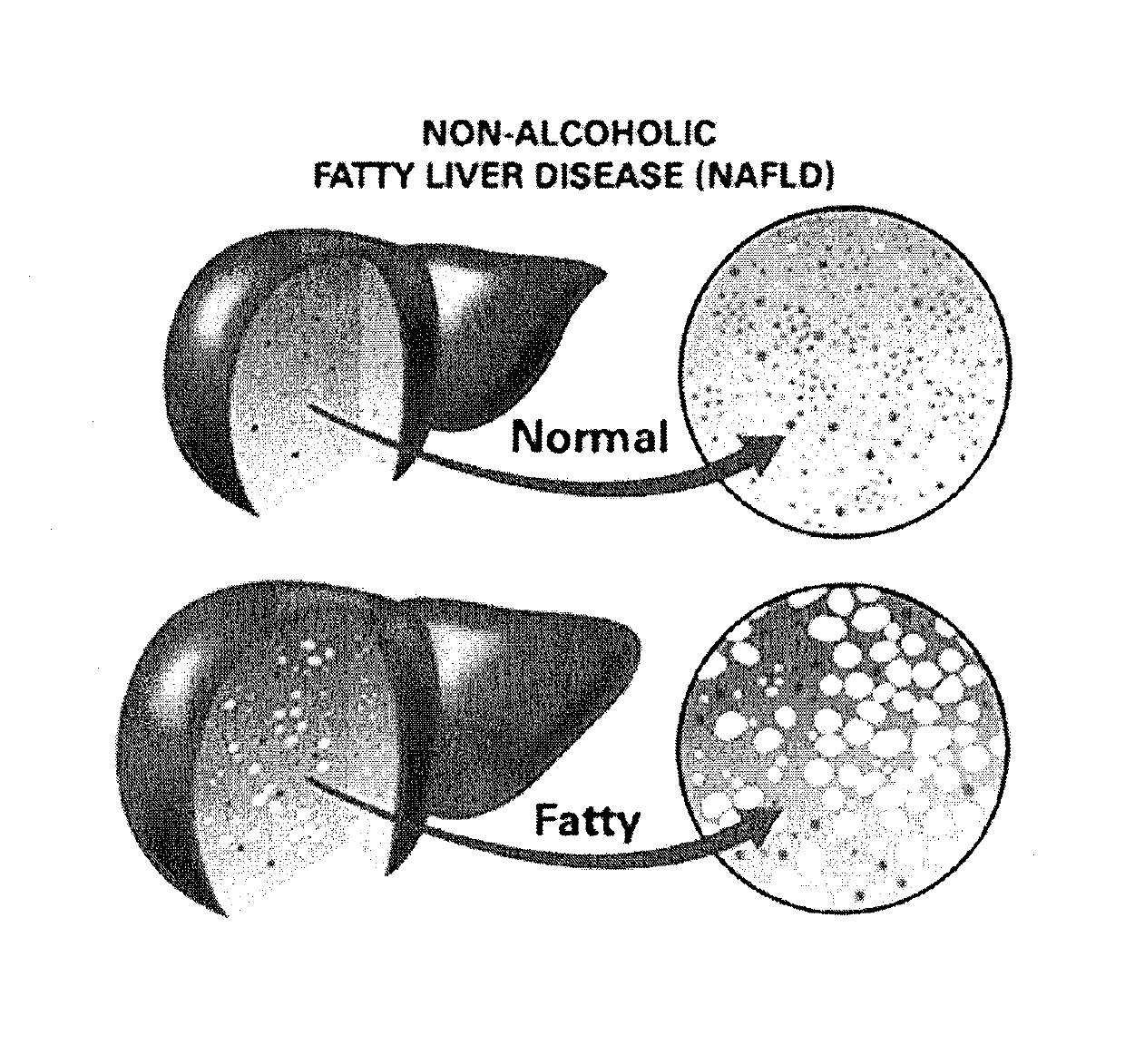
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
Nano-magnetic powder antihuman lirer cancer single anti HAb18 target medicine for magnetic heat therapy
InactiveCN1785430AWith active targetingHas long cycle stabilityPowder deliveryInorganic non-active ingredientsSide effectCancer cell
A magnetic nanoparticle-human liver cancer resistant monoclonal antibody Hab18 or its fragment Hab18F (abí»)2 or Hab18Fab as the target medicine in the form of injection for preventing and treating the liver cancer, stomach cancer and intestinal cancer by magneto-thermal therapy is proportionally prepared from magnetic nanoparticles and said antibody. Under the action of magnetic field, it can generate the heat 10-7000 W / gFe to kill cancer cells.
Owner:南京伍盛纳米科技有限公司
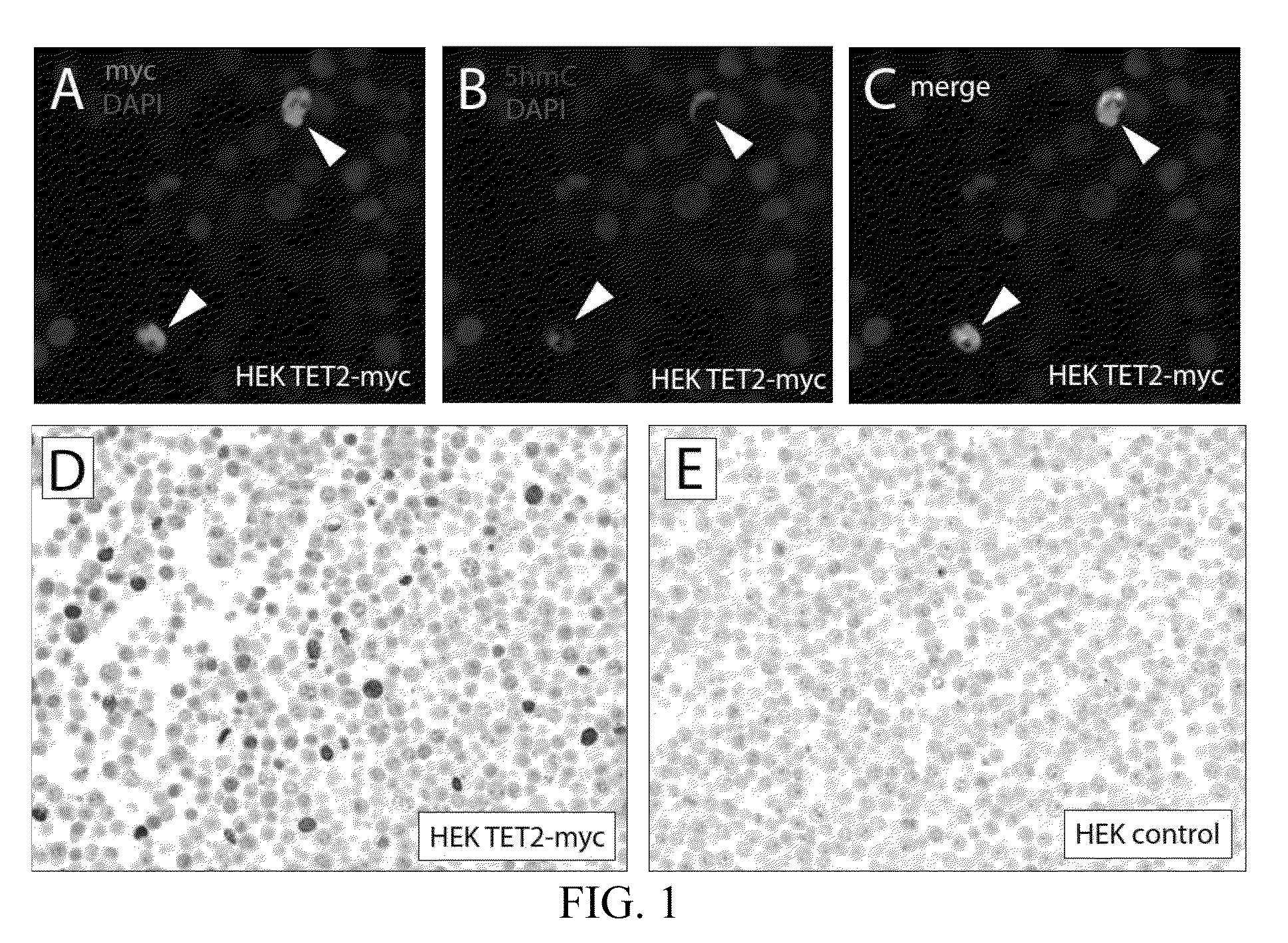
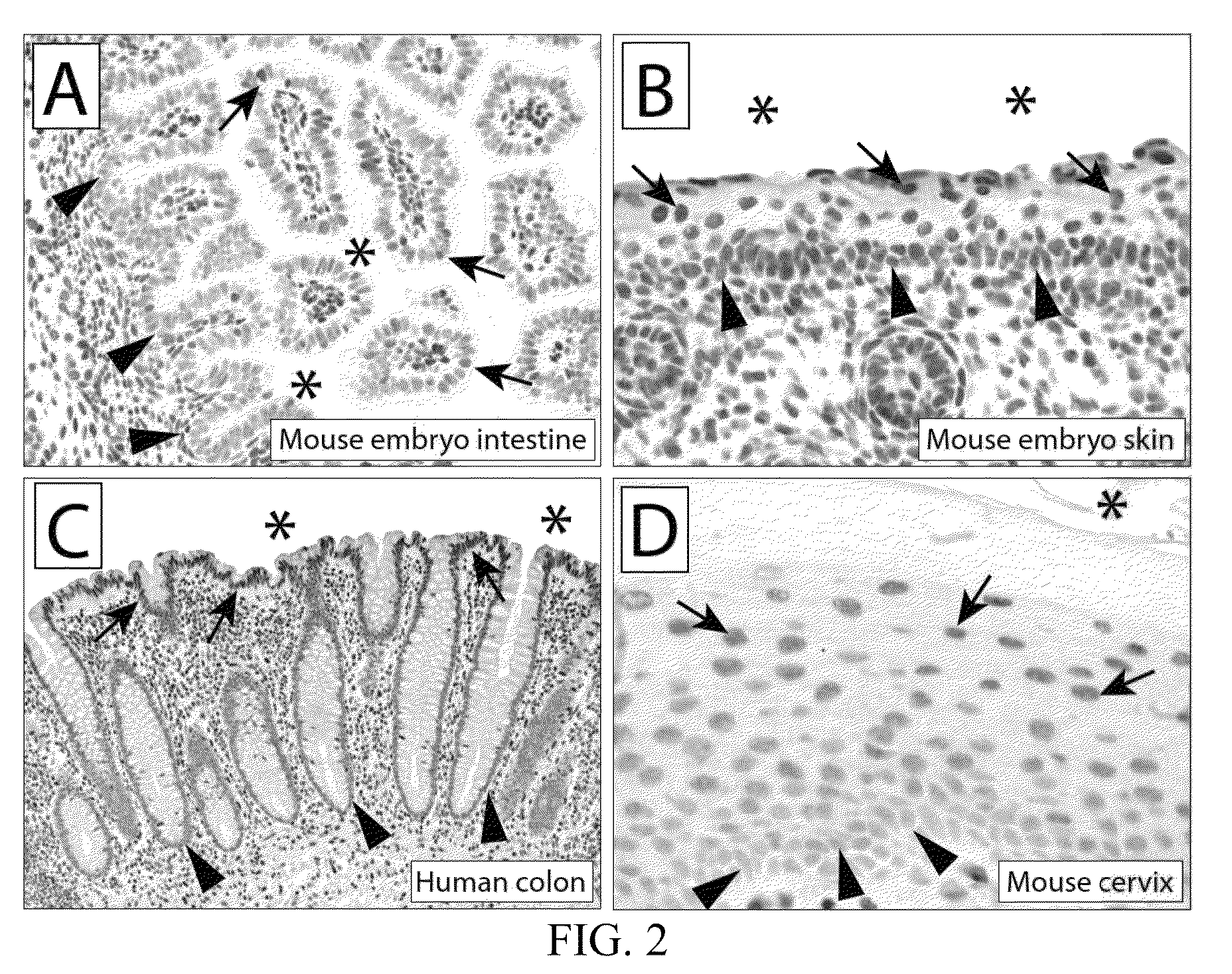
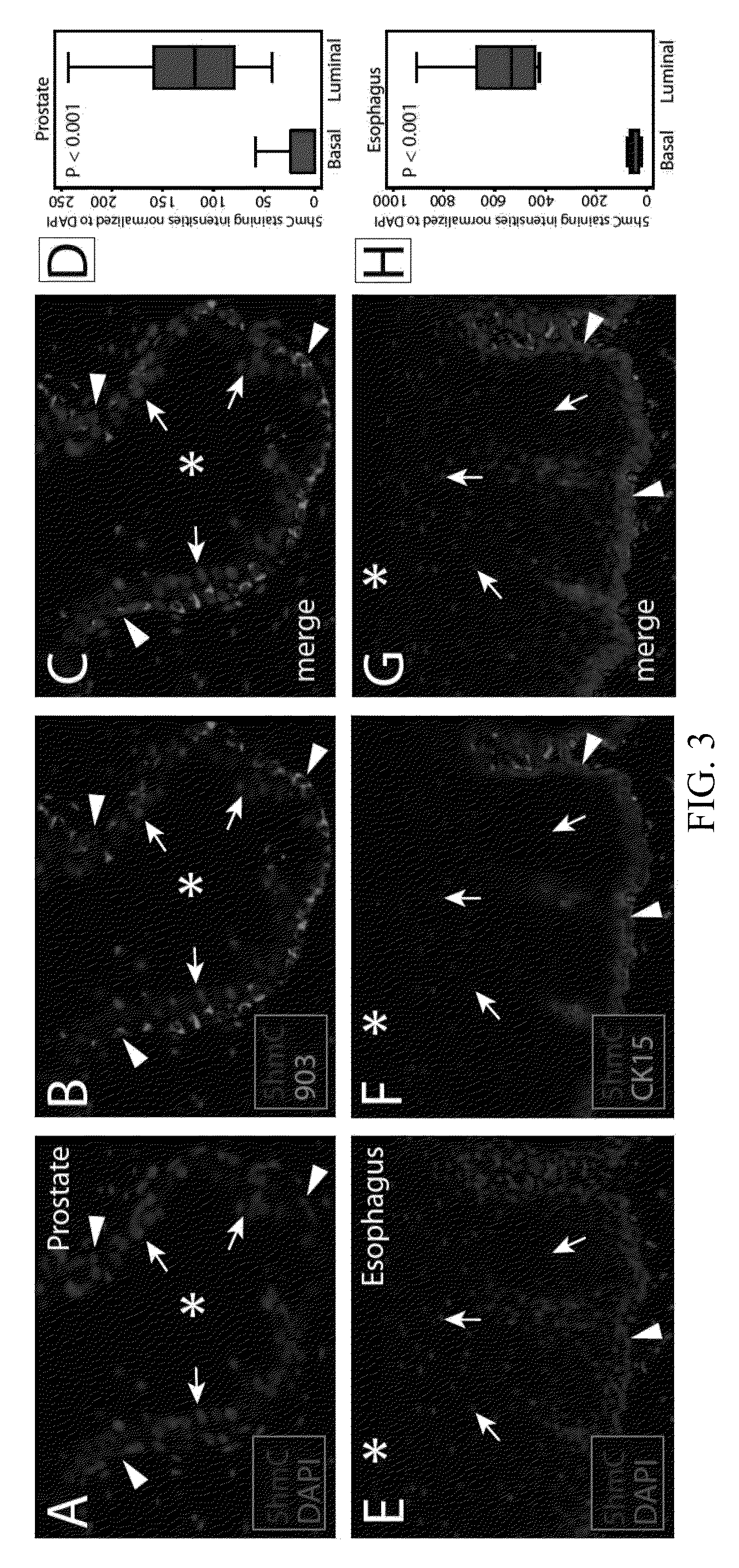
5-hydroxymethylcytosine in human cancer
The present invention relates to the field of cancer. More specifically, the present invention provides methods and compositions useful for diagnosing or predicting cancer in a patient. In one embodiment, a method for identifying a patient as having cancer comprises the steps of (a) providing a formalin-fixed, paraffin-embedded or fresh frozen sample of patient tissue; (b) steaming the sample in antigen retrieval buffer; (c) incubating the sample in hydrochloric acid (HCl); (d) incubating the sample with an affinity reagent specific for 5hmC under conditions to form a complex between the affinity reagent and 5-hydroxymethylcytosine (5hmC) present in the sample; (e) detecting the complexes formed between 5hmC and the affinity reagent with secondary detection reagents; (f) quantifying 5hmC levels; and (g) identifying the patient as having cancer if the 5hmC levels in the sample are reduced as compared to a control.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
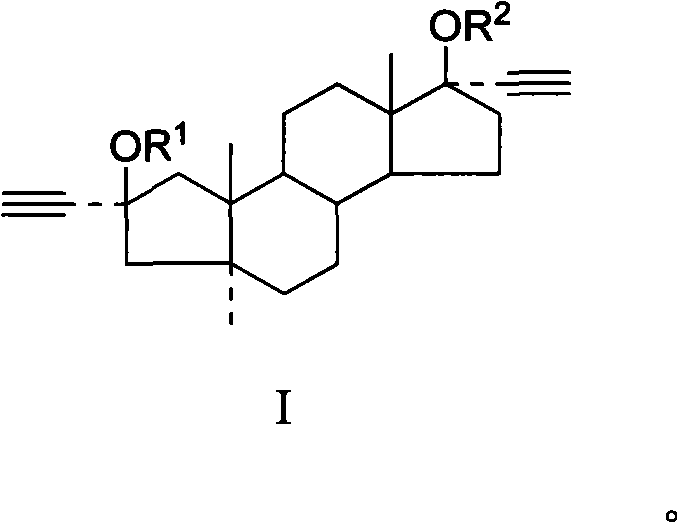
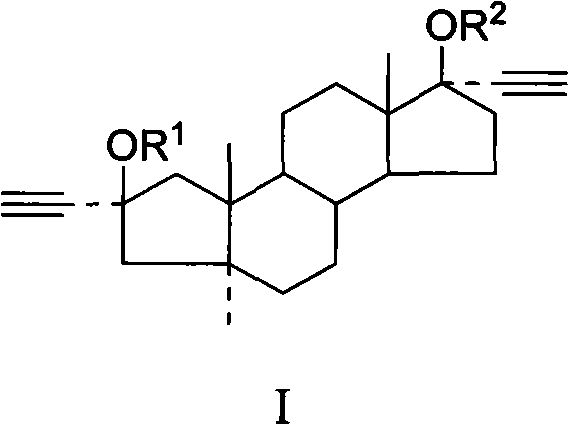
Applicationof A-nor-5 alpha-androstane compounds in preparation of malignant tumor resistant medicaments
ActiveCN102218069APotent tumor suppressor activityBroad-spectrum tumor suppressor activityOrganic active ingredientsSteroidsTreatment effectMda mb 231
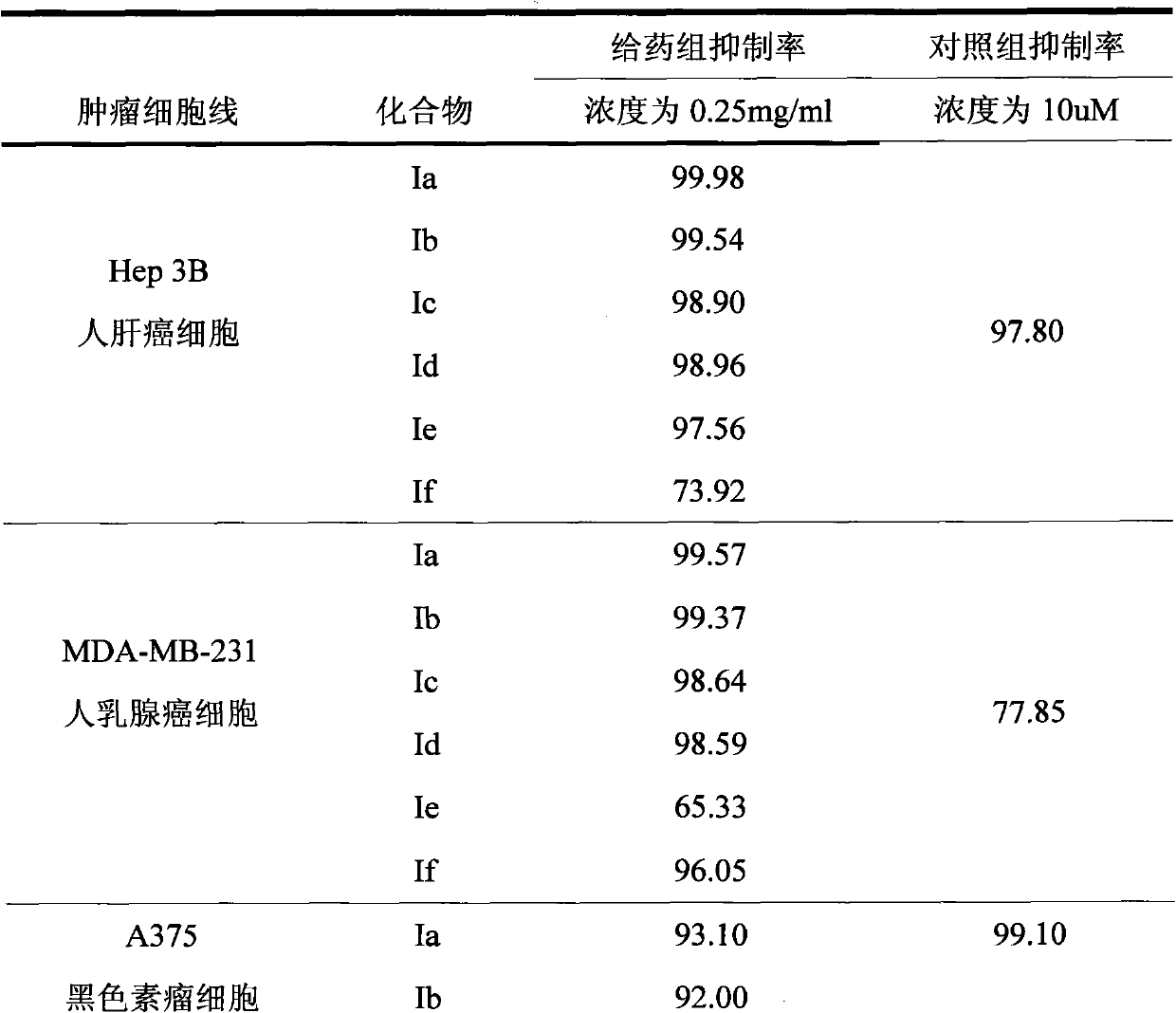
The invention discloses application of A-nor-5 alpha-androstane compounds in preparation of malignant tumor resistant medicaments. The compounds have the following general formula I, and comprise Ia, Ib, Ic, Id, Ie and If. The growth inhibition rate of the A-nor-5 alpha-androstane compounds for in-vitro human liver cancer cell Hep 3B, human breast cancer MDA-MB-231, human lung adenocarcinoma A549 and mouse melanoma B16 is higher than 85% on average, and even up to 99.98% to the maximum. The in-vivo test proves that the inhibition rate of the A-nor-5 alpha-androstane compounds for mouse tumors, such as intestinal cancer C26, liver cancer H22, Lewis lung cancer, breast cancer, B16 melanoma and the like, is higher than 50% on average, and even up to 63.19% to the maximum. The result proves that the compounds disclosed by the invention have an obvious malignant tumor resistant action. The A-nor-5 alpha-androstane compounds disclosed by the invention have an obvious and broad-spectrum action on inhibiting growth of malignant tumor cells, and are novel targeted malignant tumor resistant medicaments with low drug toxicity and favorable treatment effect; and the A-nor-5 alpha-androstane compounds just specifically act on tumor cells, but not influence normal cells, thereby having a high clinical application value.
Owner:SHANGHAI AO QI MEDICAL TECH
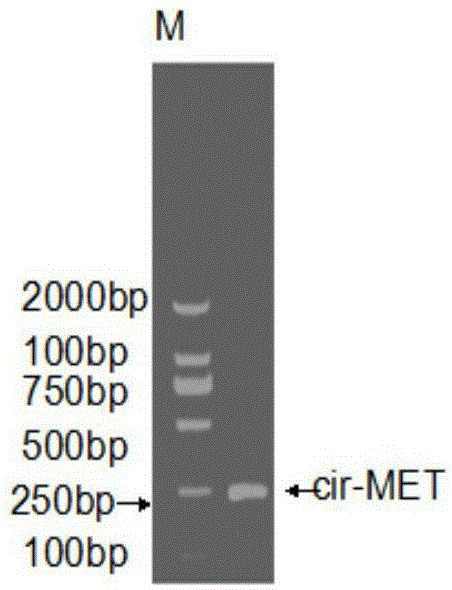
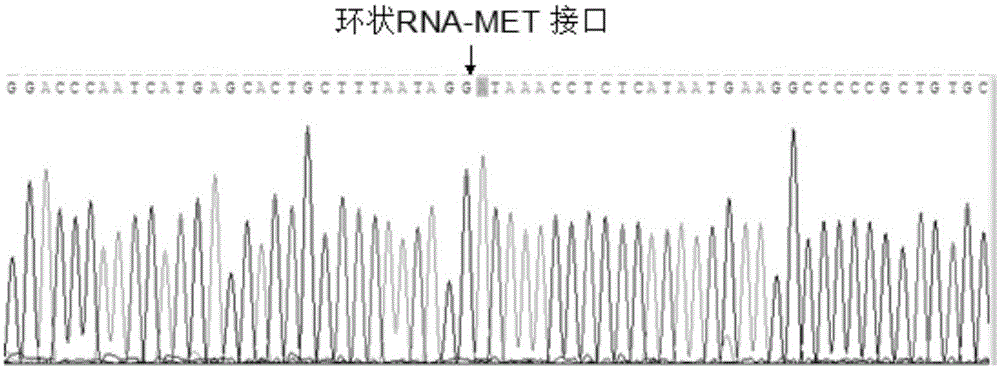
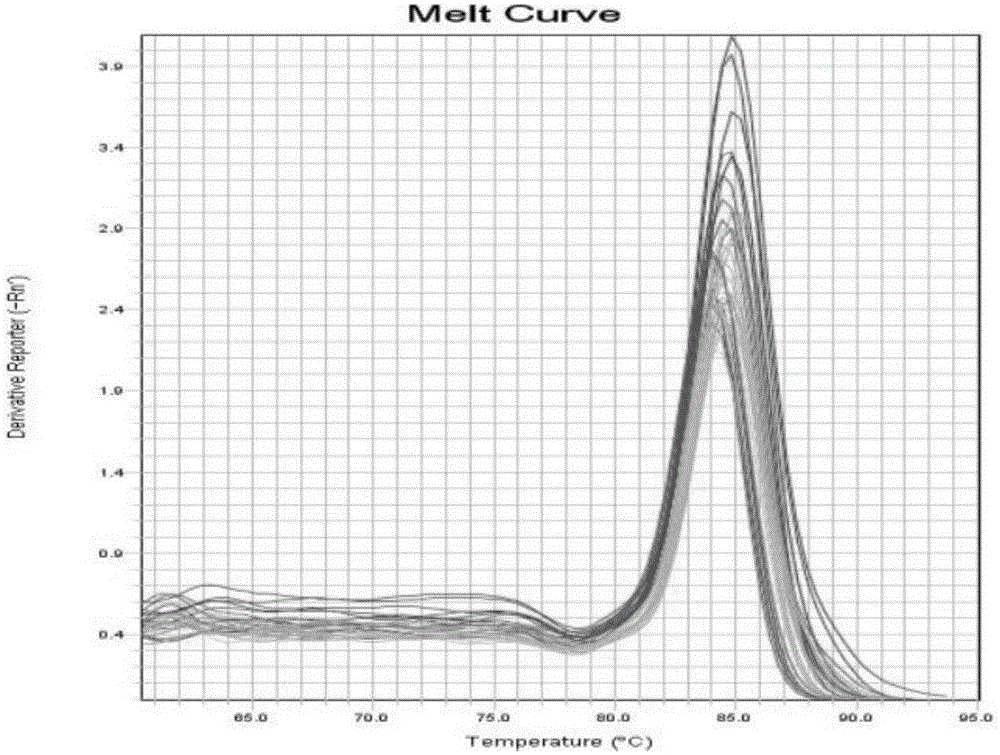
Circular RNA-MET gene in liver cancer as well as expression method and fluorescent quantitative PCR detection method of circular RNA-MET gene
ActiveCN105018495AExact cyclization siteStrong specificityMicrobiological testing/measurementPeptidesNucleotide sequencingNucleotide composition
The invention discloses a circular RNA-MET gene in liver cancer as well as an expression method and a fluorescent quantitative PCR (polymerase chain reaction) detection method of the circular RNA-MET gene. The cDNA nucleotide sequence of the circular RNA-MET gene in the liver cancer is shown by SEQ ID NO:1. By designing a specific primer capable of amplifying the circular RNA, the circular RNA of the MET gene is amplified out by PCR, and an accurate cyclization site of circular RNA-MET is measured by a method for sequencing a PCR product namely cloned DNA; and the MET gene is determined to objectively contain the circular RNA consisting of 1214 nucleotides. By using the fluorescent quantitative PCR detection method of the circular RNA-MET gene in the liver cancer, the existence and expressive differences of the circular RNA-MET in samples of liver cancer cells and liver cancer clinical tissues can be specifically detected. The invention can provide an ideal gene detection method for early clinical diagnosis of liver cancer.
Owner:GUANGZHOU GENESEED BIOTECH +1
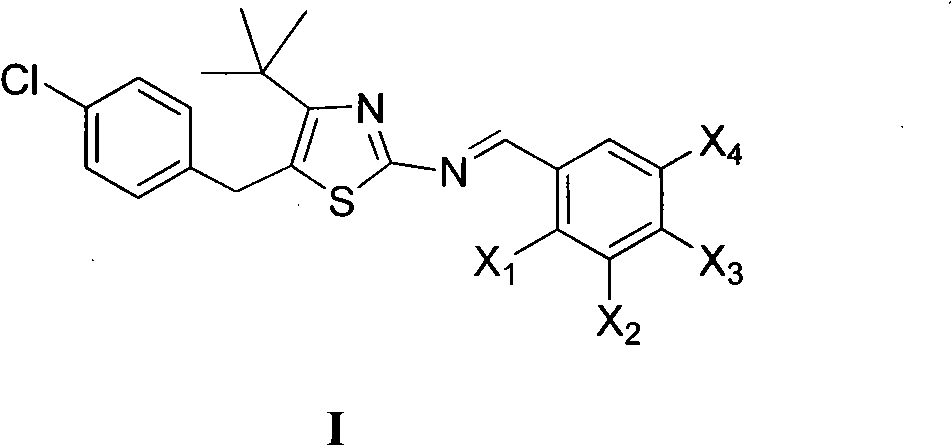
5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives and preparation method and application thereof
InactiveCN101845026AHas antitumor activityOrganic active ingredientsOrganic chemistryHydrogenBromine
The invention discloses 5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives (I), which has the following structural formula as shown in the specification of the invention, wherein X1 in a formula I is selected from hydrogen, hydroxyl group, methoxy group, nitro group, amino group and chlorine; X2 is selected from hydrogen, nitro group, amino group, methoxy group, chlorine, bromine and iodine; X3 is selected from hydrogen, methyl group, ethyl group, nitro group, methoxy group, chlorine, bromine, amino group and dimethylamino group; and X4 is selected from hydrogen, nitro group, amino group, methoxy group, chlorine, bromine and iodine. The 5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives (I) have favorable inhibition activity on human cervical cancer cells, human liver cancer cells, human nasal and oral cancer cells and the like and can be used for preparing anti-tumor medicines.
Owner:HUNAN UNIV
Targetted polypeptide for specificity of liver cancer blood vessel
InactiveCN1552727APeptide/protein ingredientsDigestive systemRandom Peptide LibraryVascular endothelium
A liver cancer blood vessel specific target polypeptide LCI-X7 able to specific binding with liver cancer blood vessel is extracted from the naked mouse model of human liver cancer by the showing technique of phage random peptide library in body and microscopic laser cutting technique. It provides important theory and practical basis for the diagnosis to liver cancer transfer recurrence and target therapy.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Marine bacillus and its polypeptide with antitumor activity
InactiveCN103224898AImprove thermal stabilityStrong cytotoxicityBacteriaPeptide/protein ingredientsHuman gliomaCytotoxicity
The invention relates to a marine bacillus and its polypeptide with antitumor activity. The strain is preserved at China Center for Type Culture Collection in Wuhan, and its preservation number is CCTCC M 2013063. A novel marine bacillus polypeptide with anti-tumor activity is obtained from a fermentation product of the strain. By means of an MTT method, the invention finds that the polypeptide has an obvious proliferation inhibition effect on human hepatoma carcinoma cell BEL-7402, human breast cancer cell MCF-7, human glioma cell U251, human non-small-cell lung cancer cell A549 and other tumor cells, has relatively small cytotoxicity on human fibroblast HFL1, and can be applied in drugs treating human liver cancer, gliomas, lung cancer and breast cancer.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Chinese medicinal powder for treating liver cancer, lung cancer and pancreatic cancer
ActiveCN102008685AFormulation ScienceSimple processPowder deliveryDigestive systemRubia sylvaticaBarbed Skullcap Herb
The invention discloses Chinese medicinal powder for treating liver cancer, lung cancer and pancreatic cancer. The Chinese medicinal powder comprises the following raw materials by weight: 14 to 16g of root of red-rooted salvia, 7 to 9g of lightyellow sophora root, 9 to 11g of largehead atractylodes rhizome, 14 to 16g of common leafflower herb, 8 to 9g of Chinese sage herb, 9 to 11g of mahonia fortunei, 9 to 11g of glabrous greenbrier rhizome, 9 to 11g of heartleaf houttuynia herb, 9 to 11g of buttercup root, 9 to 11g of herba geranii, 9 to 11g of stringy stonecrop herb, 19 to 21g of buckwheat, 7 to 9g of hairyvein agrimonia herb and bud, 9 to 11g of oriental waterplantain rhizome, 15g of bupleurum, 9 to 11g of mongolian snakegourd root, 9 to 11g of akebia trifoliata koidz, 14 to 16g of motherwort herb, 19 to 21g of giant knotweed rhizome, 8g of rhodiola, 9 to 11g of amur corktree bark, 38 to 42g of astragalus, 9 to 11g of baical skullcap root, 14 to 16g of rhubarb, 9 to 11g of lily, 9 to 11g of bittersweet herb, 14 to 16g of Chinese lobelia herb, 14 to 16g of barbed skullcap herb, 14 to 16g of spreading hedyotis herb, 7 to 9g of pinellia tuber, 9 to 11g of paris polyphylla, 9 to 11g of gardenia, 9 to 11g of agaric, 14 to 16g of snow of june herb, 19 to 21g of Chinese brake herb, 9 to 11g of India madder root, 9 to 11g of clematis root, 9 to 11g of gentian, 9 to 11g of Japanese climbing fern spore, 9 to 11g of Chinese pyrola herb, 9 to 11g of sinkiang arnebia root, 9 to 11g of paniculate swallowwort root, 9 to 11g of costustoot, 9 to 11g of zanthoxylum bungeanum maxim, 9 to 11g of radix stephaniae tetrandrae, 30g of glossy privet fruit, 19 to 21g of dendrobium, 14 to 16g of sweet wormwood herb, 19 to 21g of chinaroot greenbrier and 9 to 11g of zedoary. The Chinese medicinal powder has the advantages of readily available raw materials, good effect of treating cancers, quick response and high cure rate.
Owner:高学哲
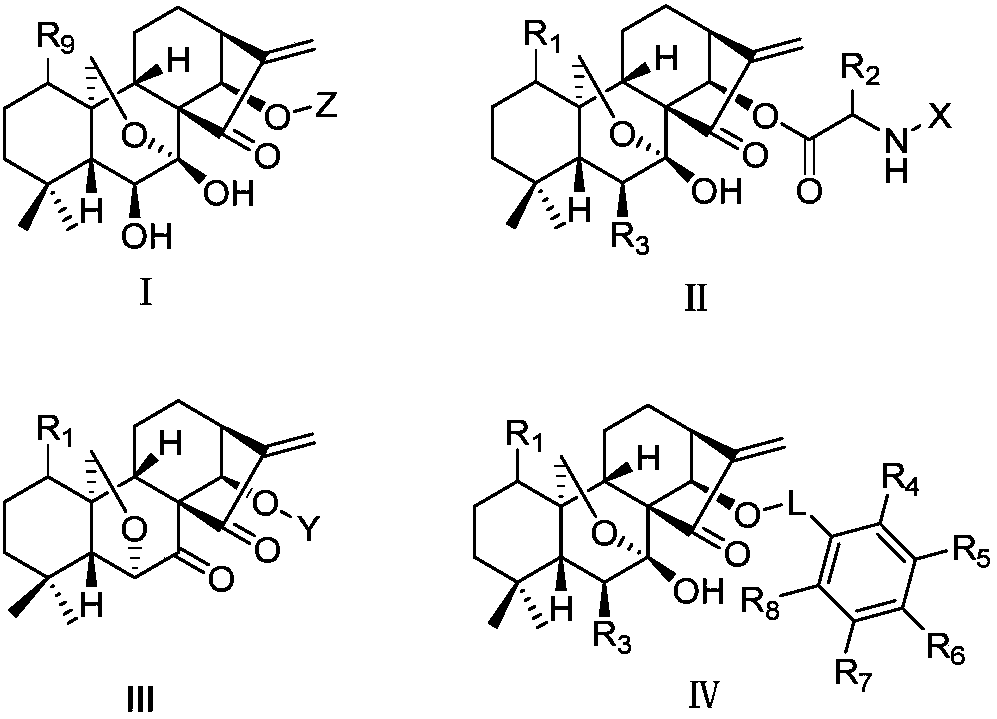
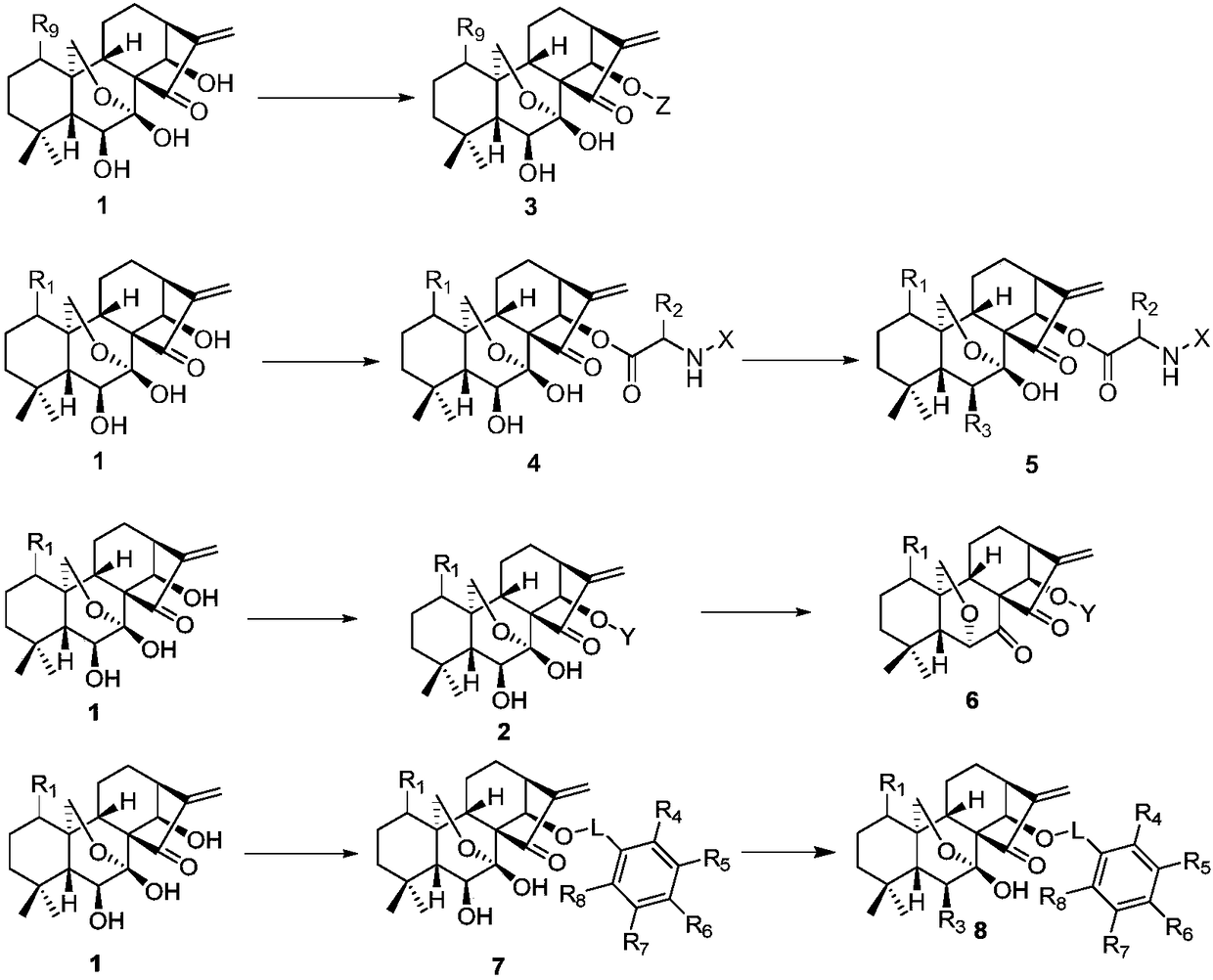
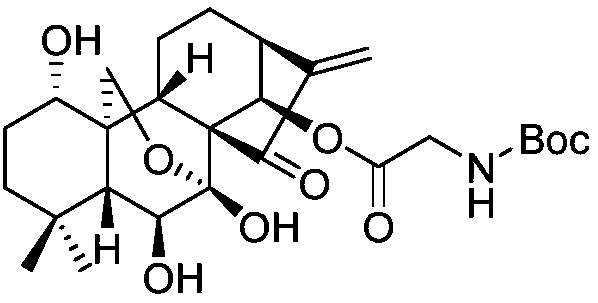
Rubescensin A derivatives and preparation method and applications thereof
The invention discloses rubescensin A derivatives and a preparation method and applications thereof. The rubescensin A derivatives have structures represented by formulas (I), (II), (III), or (IV). Onthe premise that the active center of rubescensin A is not destroyed, the hydroxyl groups on the 1st, 6th, and 14th positions or the hydroxyl groups on the 6th and 7th positions are subjected to ringchange modification; and obtained rubescensin A derivatives have higher antitumor activity, higher intercellular selectivity, lower dosage, and lower toxicity. Compared with that of rubescensin A, the performance of rubescensin A derivatives on killing cancer cells of human liver cancer, human multiple myeloma, human lung cancer, and the like, is enhanced by 3 to 5 times; the optimal performancecan be strengthened by 11 times; and the rubescensin A derivatives can be used to prepare antitumor drugs for treating liver cancer, rubescensin A derivatives, lung cancer, and the like.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD +1
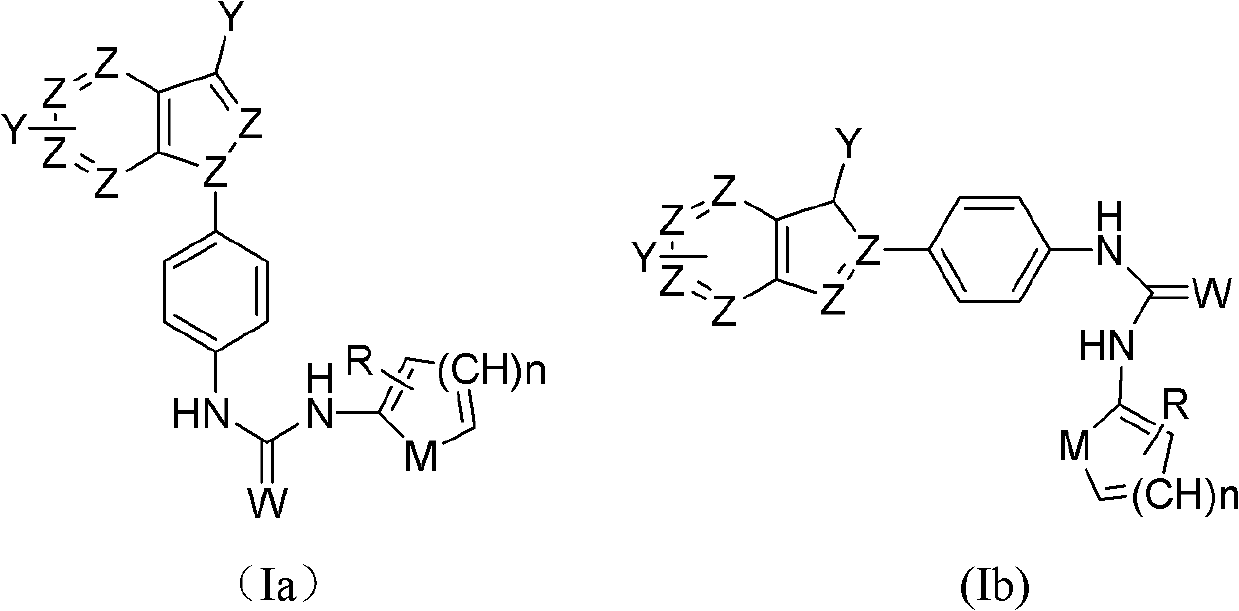
Indazole/azaindazole-based diarylcarbamide/thiocarbamide-structure antineoplastic drug
InactiveCN102153551AOvercoming drug resistanceOrganic active ingredientsSulfonic acids salts preparationNitrosoKetone
The invention belongs to the field of drugs, and relates to an antineoplastic drug, particularly an indazole / azaindazole-based diarylcarbamide / thiocarbamide-structure antineoplastic drug. The structural general formulae of the indazole / azaindazole-based diarylcarbamide / thiocarbamide-structure antineoplastic drug are disclosed as Formula (Ia) and (Ib), wherein Z is an N or C atom; W is an atom or group, such as O, S, NH, NOH, NCH or the like; M is O, S, N, CH or the like; n is 1 or 2; and Y and R are respectively halogen atom, H, R1, CF3, OCF3, OH, OR2, OCOR3, NH2, NHR4, NR52, NHCOR6, carboxy group, ester group, cyano-group, sulfhydryl group, alkylthio group, sulfuryl group, sulfoxide group, sulfo-group, sulfonate group, sulfamide group, ketone group, aldehyde group, nitro-group or nitroso-group. The pharmacological experiment proves that the drug has favorable antineoplastic effect on human lung cancer, human kidney cancer, human colon cancer, human liver cancer, human stomach cancer and human breast cancer.
Owner:JINAN HAILE MEDICAL TECH DEV
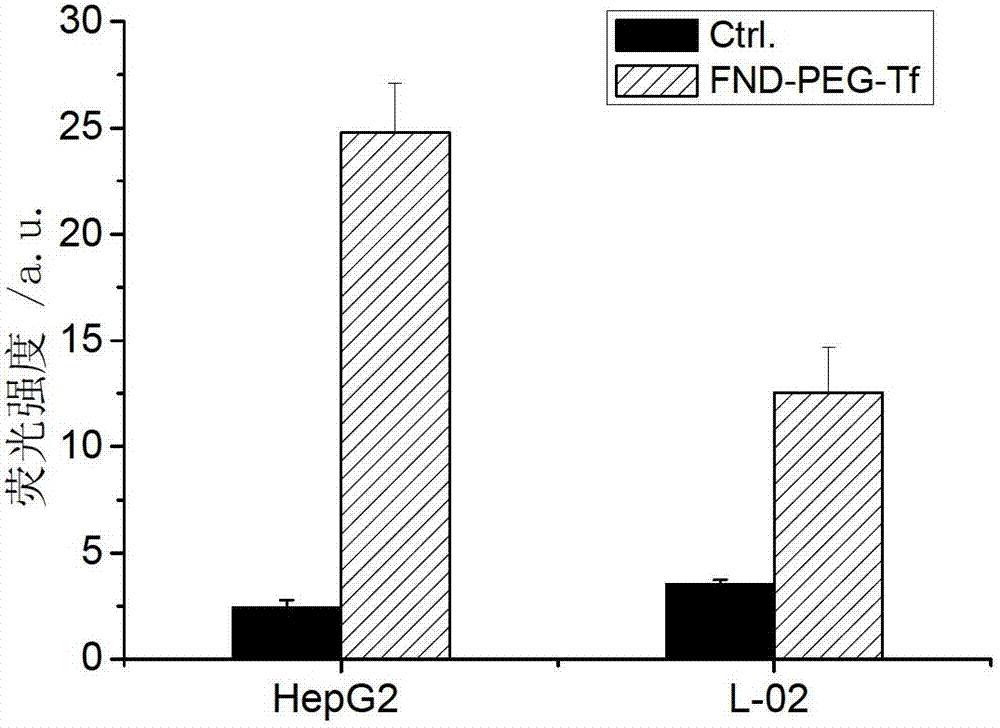
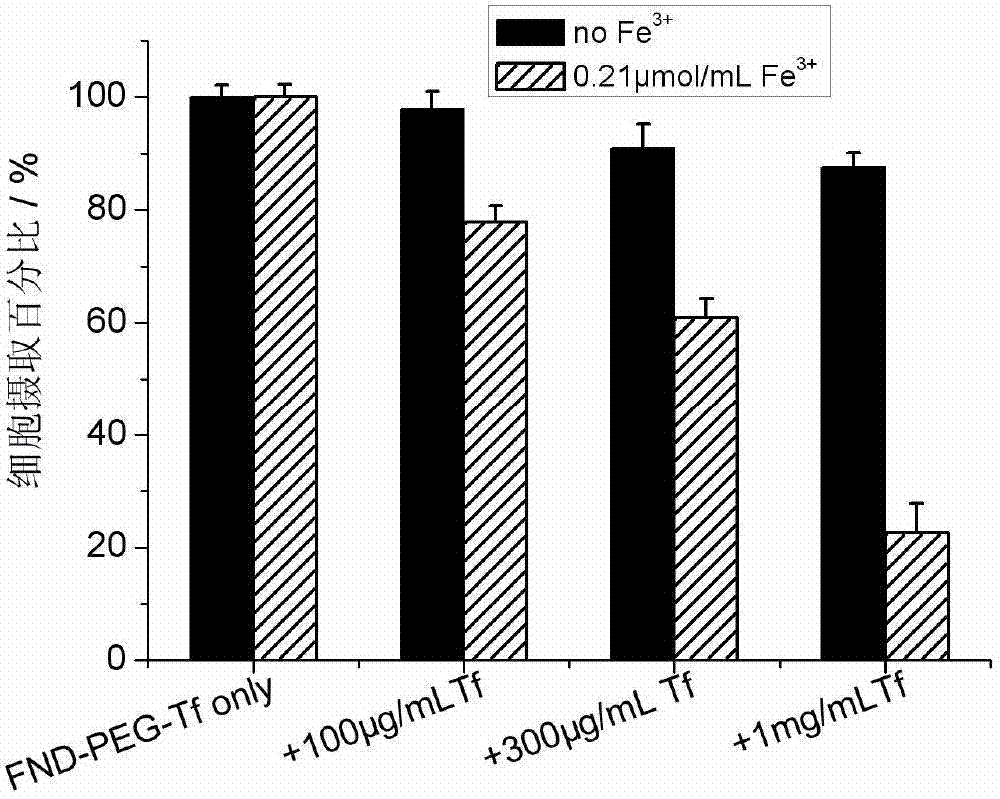
Targeting nano diamond carrier and targeted drug, and preparation method and application thereof
InactiveCN103203024AGood biocompatibilityNo toxicityOrganic active ingredientsAntiinfectivesSide effectCancer cell
The invention provides a targeting nano diamond carrier ND-PEG-Tf and a preparation method, and a targeted nano-drug ND-PEG-Tf-DOX prepared by loading chemotherapeutic adriamycin through physical absorption. The carrier interacts with a human liver cancer cell (HepG2) and a normal liver cell (L-02) and free transferrin is taken as a competitor, and the result indicates that the ND-PEG-Tf carrier is mediated by a transferrin receptor into the cells; MTT testing of interaction of ND-PEG-Tf-DOX with the two cells indicates that the nano-drug has targeted anti-tumor effect on cancer cells; and compared with the cell damage force of independent adriamycin, ND-PEG-Tf-DOX has the characteristics of a slow-release drug. Therefore, ND-PEG-Tf-DOX is capable of either reducing toxic and side effects of chemotherapeutics on normal cells or improving the damage force of the chemotherapeutics on tumor cells. In addition, the targeting nano diamond carrier can be applied to preparing targeted anti-tumor drugs.
Owner:SHANXI UNIV
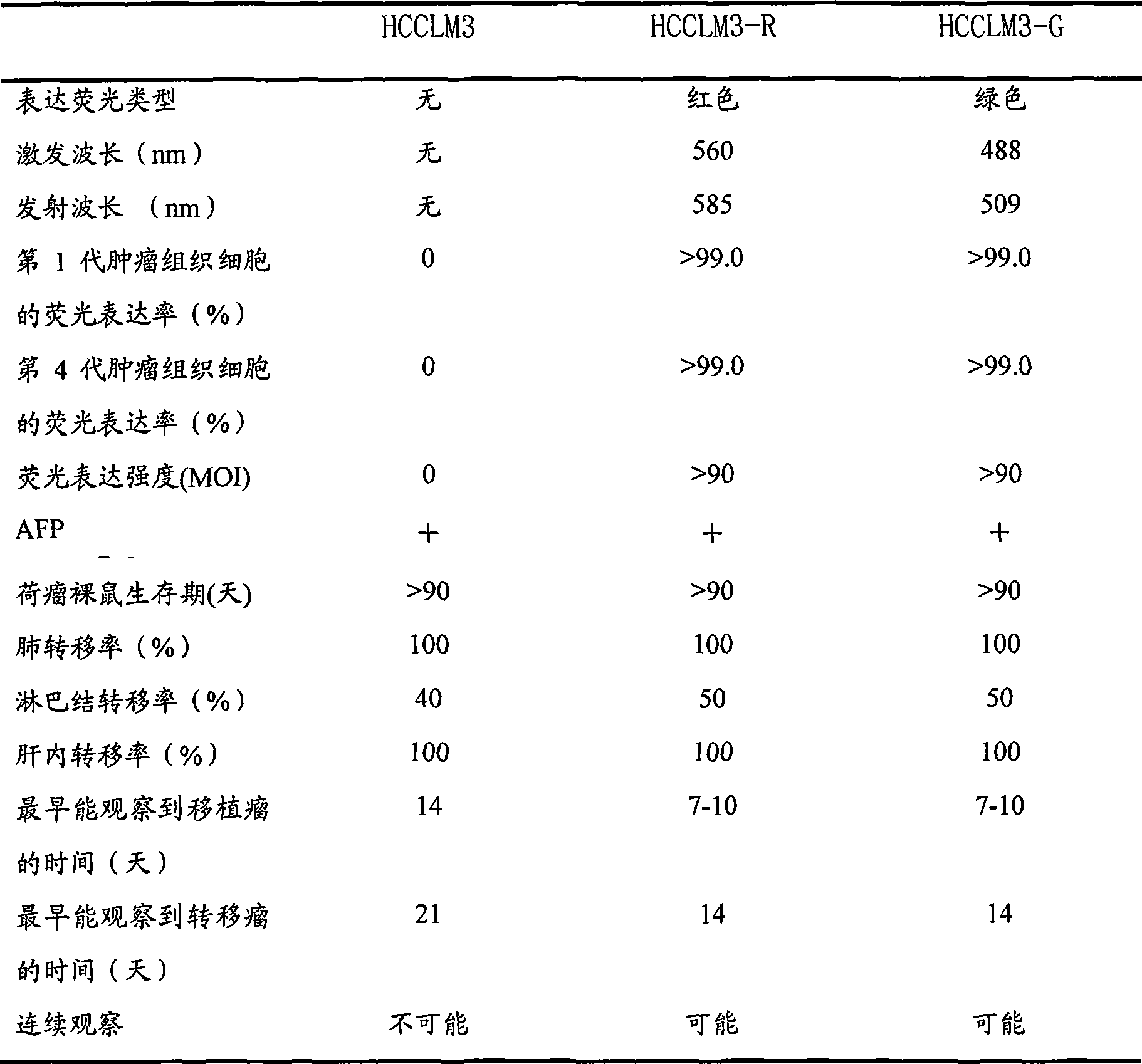
Human liver cancer high-transfer cell strain with stable expression of fluorescent protein and construction method thereof
InactiveCN101381706AAvoid pollutionImprove efficiencyVector-based foreign material introductionForeign genetic material cellsCarcinoma cell lineCancer cell
The invention belongs to the field of micro-organism animal cell line and relates to a human hepatoma cell line which can emit high-intensity red or green fluorescence and has high transferring ability of lung and lymph node metastasis, and a method for establishing the same. The method comprises the following steps: using the human hepatoma cell line HCCLM3 and HCCLM6 which have high transferring ability of the lung and lymph node metastasis as mother cells, performing cotransfection on plasmid DNA of 239 cells through slow virus packaging plasmids to obtain false slow virus particles by expressing red or green fluorescent protein genes through eucaryon, and infecting liver cancer cell strains of the mother cells to obtain the chromosome integrated hepatoma cell line which has high transferring ability of the lung and lymph node metastasis and can stably expressing the red or the green fluorescence. The human hepatoma cell line which has high transferring ability of the lung and lymph node metastasis in vitro can be applied to the tracer studies on tumor cells, the molecular mechanism studies on the recurrence and transferring of liver cancer, as well as the pre-clinical drug efficacy studies on new anti-tumor drugs, thus the human hepatoma cell line has wide application prospect.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Mutant ROS Expression In Human Cancer
ActiveUS20110287445A1Drive proliferationDrive survivalOrganic active ingredientsPeptide/protein ingredientsNucleotideADAMTS Proteins
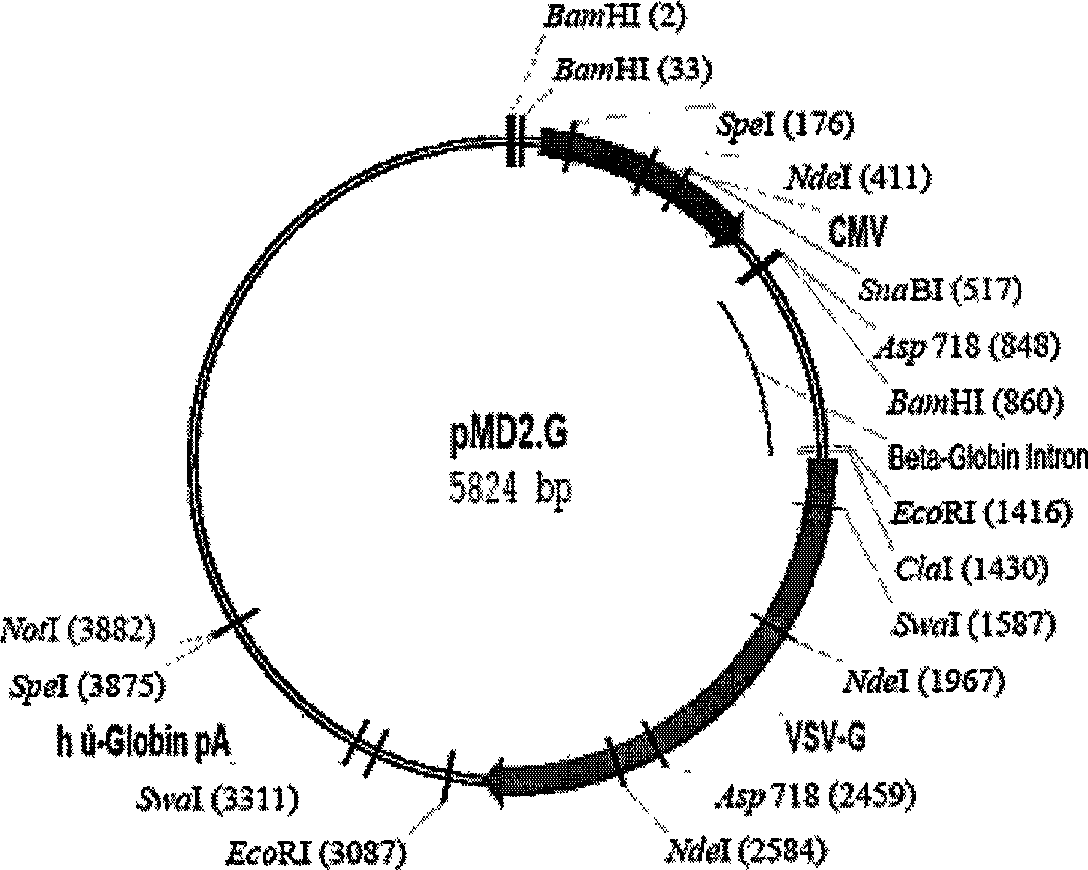
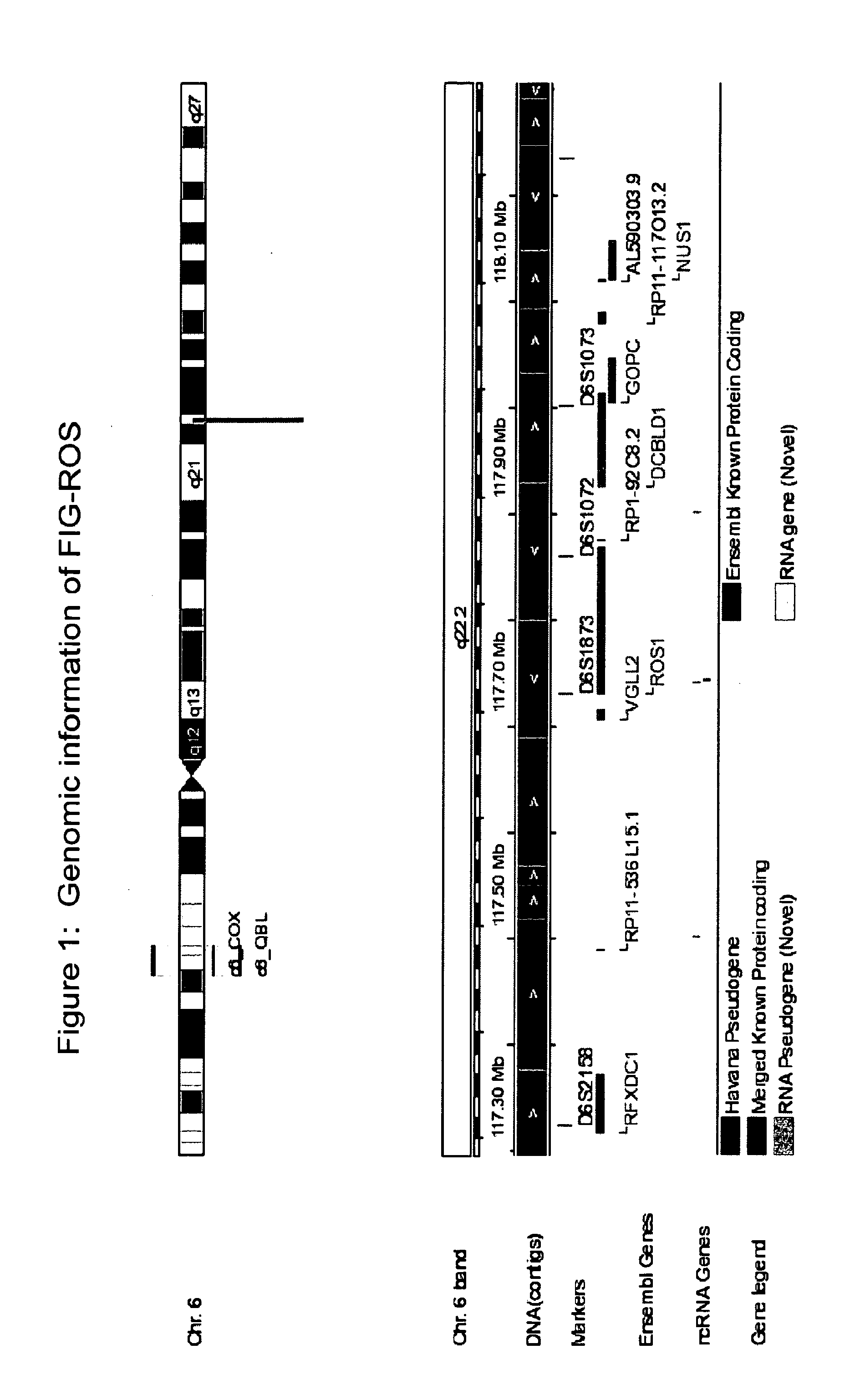
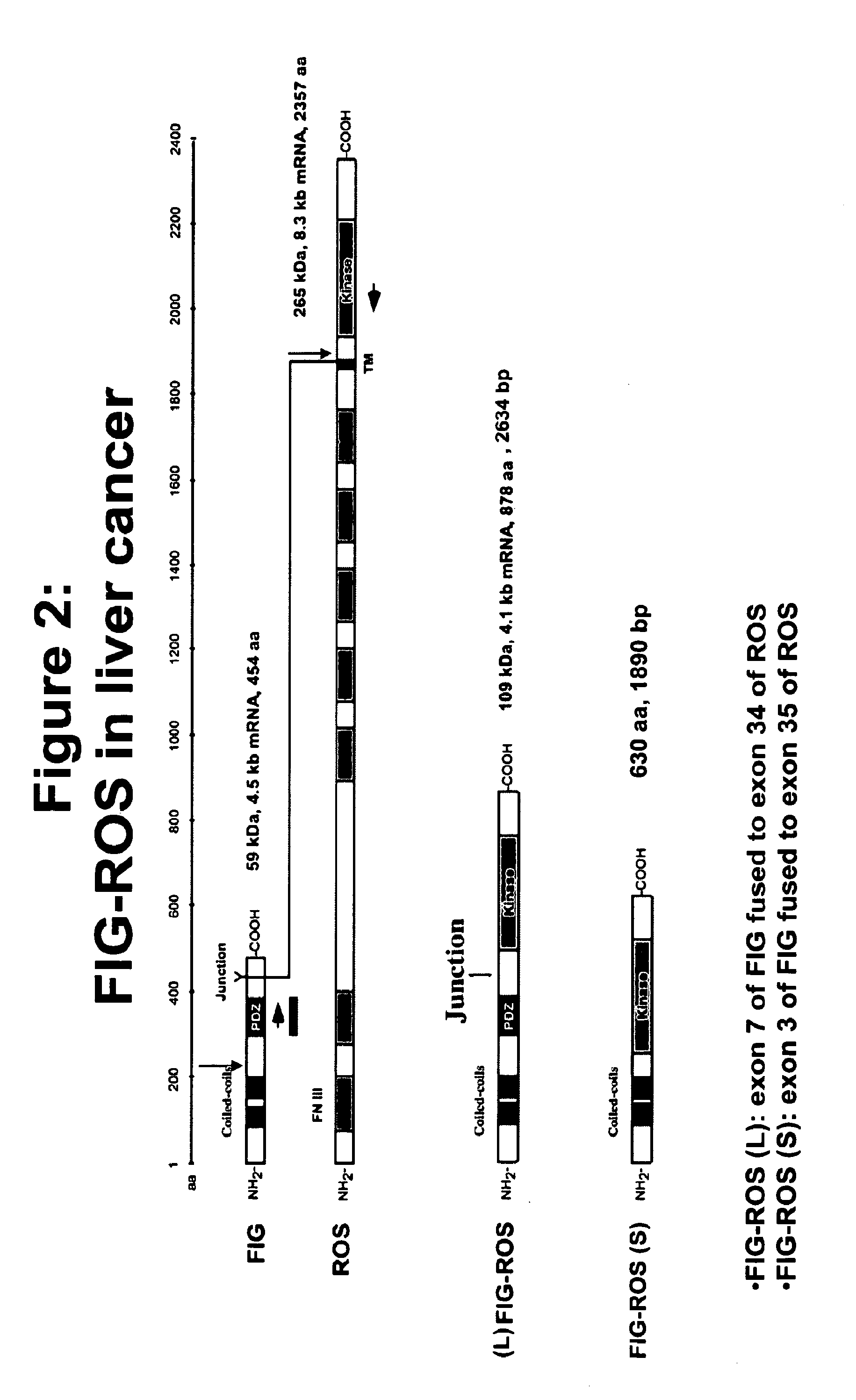
The invention provides the identification of the presence of mutant ROS protein in human cancer. In some embodiments, the mutant ROS are FIG-ROS fusion proteins comprising part of the FIG protein fused to the kinase domain of the ROS kinase. In some embodiments, the mutant ROS is the overexpression of wild-type ROS in cancerous tissues (or tissues suspected of being cancerous) where, in normal tissue of that same tissue type, ROS is not expressed or is expressed at lower levels. The mutant ROS proteins of the invention are anticipated to drive the proliferation and survival of a subgroup of human cancers, particularly in cancers of the liver (including bile duct), pancreas, kidney, and testes. The invention therefore provides, in part, isolated polynucleotides and vectors encoding the disclosed mutant ROS polypeptides (e.g., a FIG-ROS(S) fusion polypeptide), probes for detecting it, isolated mutant polypeptides, recombinant polypeptides, and reagents for detecting the fusion and truncated polypeptides. The identification of the mutant ROS polypeptides enables new methods for determining the presence of these mutant ROS polypeptides in a biological sample, methods for screening for compounds that inhibit the proteins, and methods for inhibiting the progression of a cancer characterized by the mutant polynucleotides or polypeptides, which are also provided by the invention.
Owner:CELL SIGNALING TECHNOLOGY
Blood miRNA marker related to liver cancer and application thereof
InactiveCN104152452AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMirna microarray
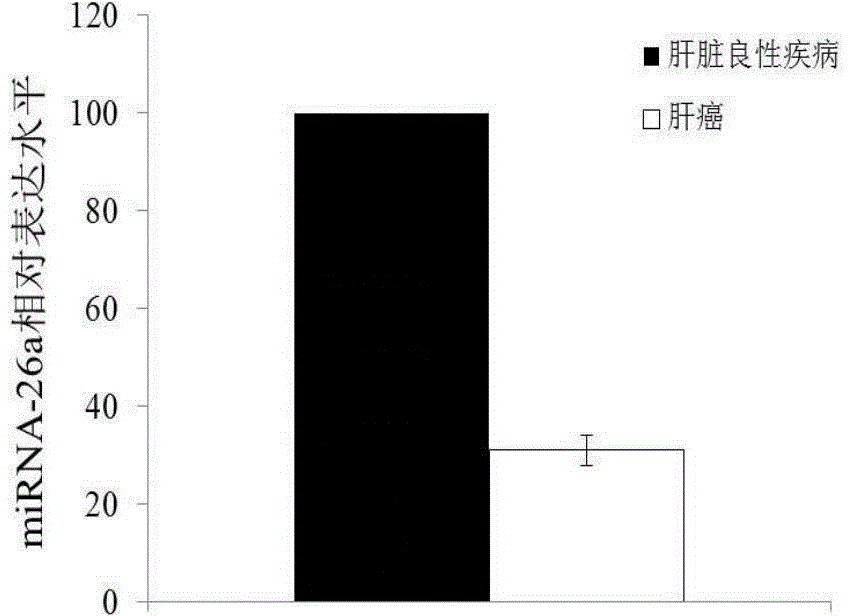
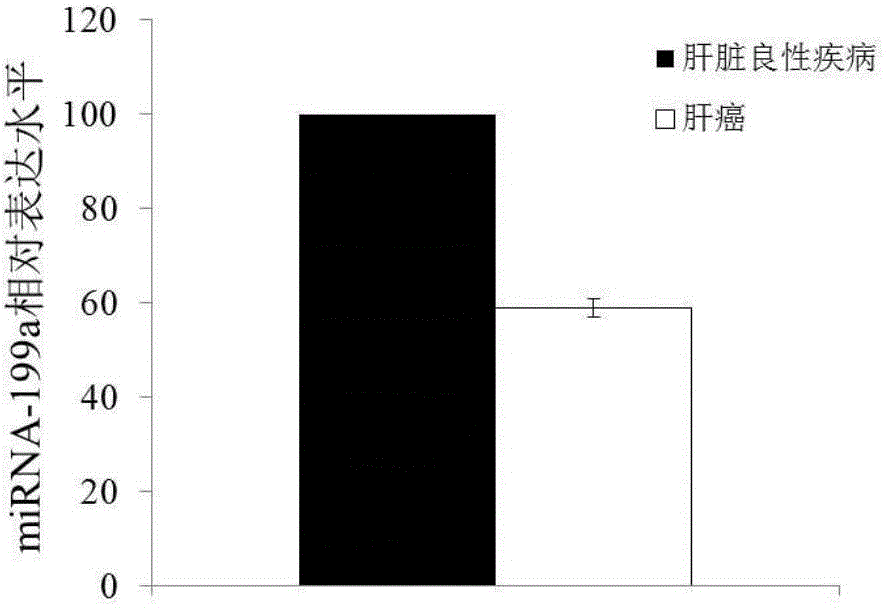
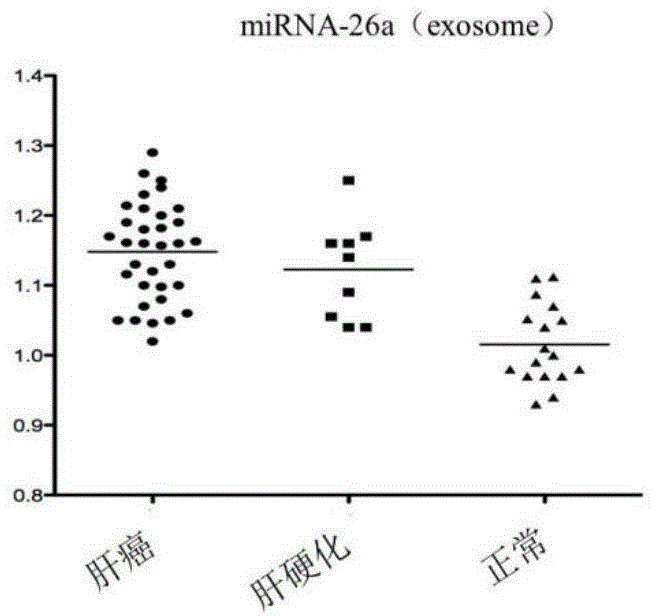
The invention discloses a blood miRNA marker related to liver cancer and an application thereof. The marker is the combination of tumor-induced exosome sourced miRNA-26a and miRNA-199a in human blood. According to the invention, tumor-induced exosome in peripheral blood of liver cancer patient and liver benign disease patient can be separated and purified, miRNA of two expression difference can be analyzed and screened by a miRNA chip, and the above conclusion is passed by a QPCR verification. The marker and its detection reagent can be used for preparing a diagnostic kit, which can be used for early diagnosis of liver cancer.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
1,3-substituted-5-acetaminoindolone compounds and application thereof to anti-tumor drugs
ActiveCN103554008AHas anti-tumor biological activitySimple operation processOrganic active ingredientsOrganic chemistryHuman leukemiaKetone
The invention relates to 1,3-substituted-5-acetaminoindolone compounds and an application thereof to anti-tumor drugs. The compounds are 1-methyl-5-acetamino-2-indolinone, 1-(4-bromobenzyl)-5-acetamino-2-indolinone, 1-(4-methylbenzyl)-3-oxime-5-acetaminoindolone, 1-(4-methoxybenzyl)-3-oxime-5-acetaminoindolone and the like. The in-vitro tumor cell inhibitory activities of the 1,3-substituted-5-acetaminoindolone compounds synthesized in the invention are tested and the results show that such kind of compounds have certain inhibiting effects (IC50(100mu M)) on human leukemia cells (K562), human colon cancer cells (HT-29) and human liver cancer cells (HepG2), have anti-tumor activities and can be used for preparing anti-tumor drugs.
Owner:无锡珉琰管理咨询服务有限公司
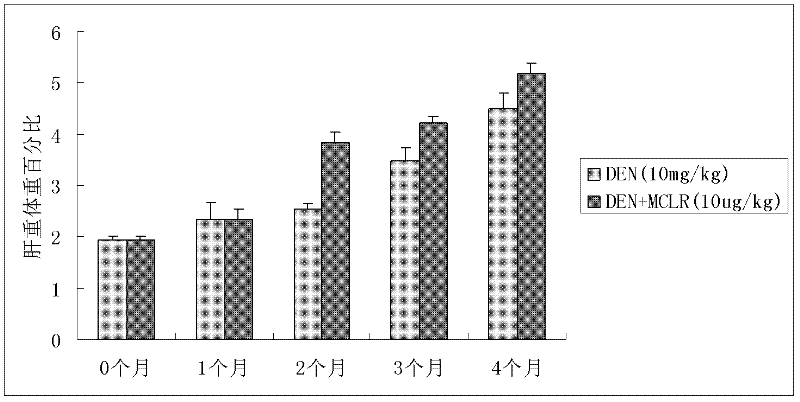
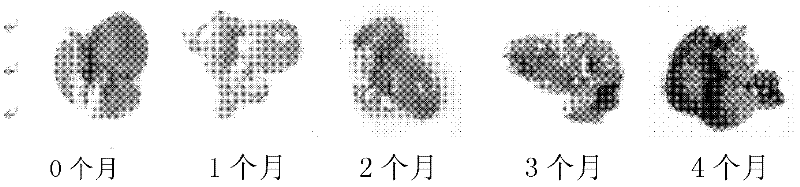
Method for establishing microcystin MC-LR promoted diethyl nitrosamine DEN induced rat liver cancer model
InactiveCN102524180ACyclic peptide ingredientsVeterinary instrumentsIntraperitoneal routeProcess mechanism
The invention discloses a method for inducing a rat liver cancer model by injecting diethyl nitrosamine DEN in coordination with microcystin MC-LR into a rat body through intraperitoneal injection. Indexes such as liver weight to body weight ratio, liver appearance, liver histopathological slicing and GST-Pi (Glutathione S-transferase) protein expression level clearly show liver cell injury phase, liver cell hyperplasia-hepatocirrhosis phase and liver cell carcinomatous change process in a rat liver cancer occurring process, and find that the liver cancer model induced by the DEN in coordination with the microcystin MC-LR has a more obvious effect at the same time than that induced by DEN independently; the tumor formation time is shorter; and the model is an ideal model for researching occurrence of the liver cancer of a human body. The model has the characteristic of clearly showing each stage for occurrence of the liver cancer and contributing to research of a liver cancer occurrence process mechanism, detection of precancerous lesion and development of treatment medicaments.
Owner:ANHUI UNIVERSITY
Compound with antitumor activity and preparation method and application of compound
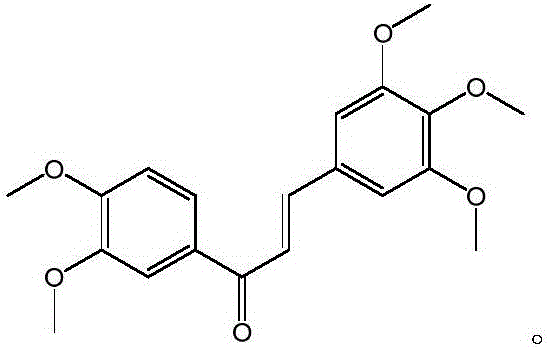
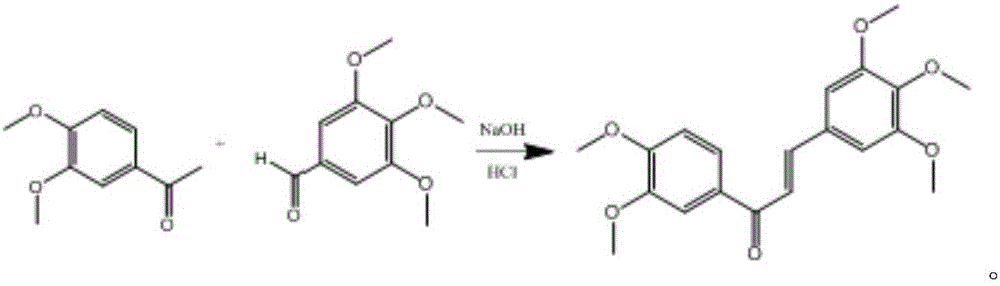
ActiveCN105967991AReduce dosageAvoid destructionOrganic compound preparationCarbonyl compound preparationChalconeTumor cells
The invention discloses a compound with antitumor activity and a preparation method and application of the compound and belongs to the technical fields of new compound synthesis and medicine application. According to the compound, the preparation and the application thereof disclosed by the invention, aromatic aldehyde and aromatic ketone are catalyzed by sodium hydroxide to synthesize 3,4,5-triethoxy-3',4'-dimethoxy chalcone for the first time, and the test of in vitro tumor cell inhibitory activity on the 3,4,5-triethoxy-3',4'-dimethoxy chalcone is realized; the result shows that the compound has higher inhibitory activity for human lung cancer cell A549, human colon carcinoma cell SW620 and human liver cancer cell HepG2. In addition, the antitumor activity of the 3,4,5-triethoxy-3',4'-dimethoxy chalcone for the human colon carcinoma cell SW620 and the human liver cancer cell HepG2 is superior to that of a reference drug 5-fluorouracil. The invention provides a new effective treatment means for tumor treatment and has a broad application prospect.
Owner:HARBIN MEDICAL UNIVERSITY
Human liver cancer marker and application thereof
InactiveCN108315413AHigh expressionImprove early diagnosis rateMicrobiological testing/measurementMaterial analysisDiagnosis earlyClinical prognosis
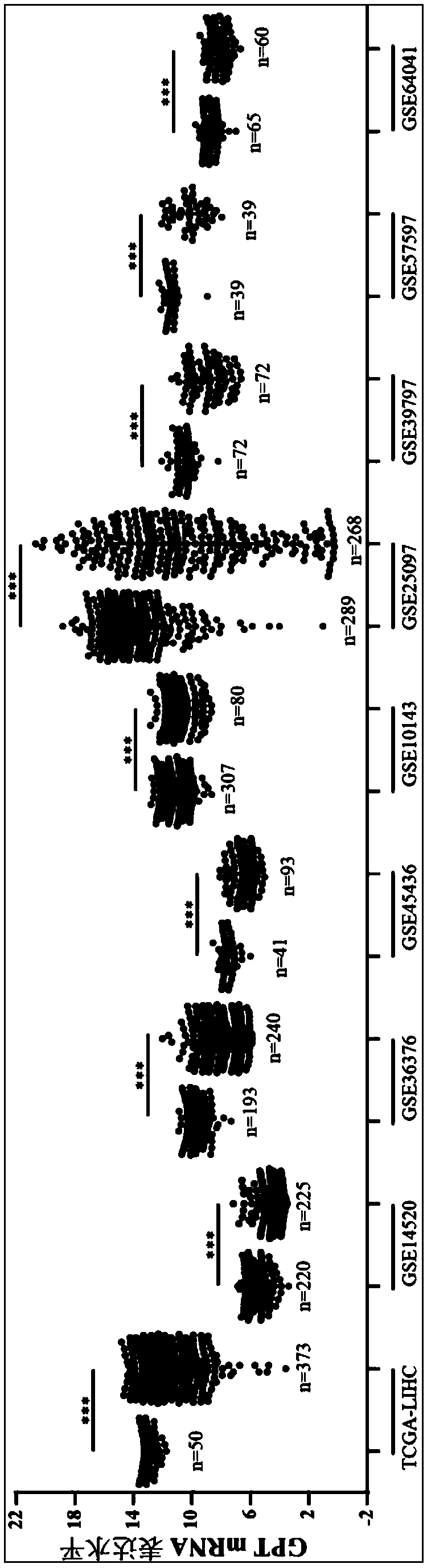
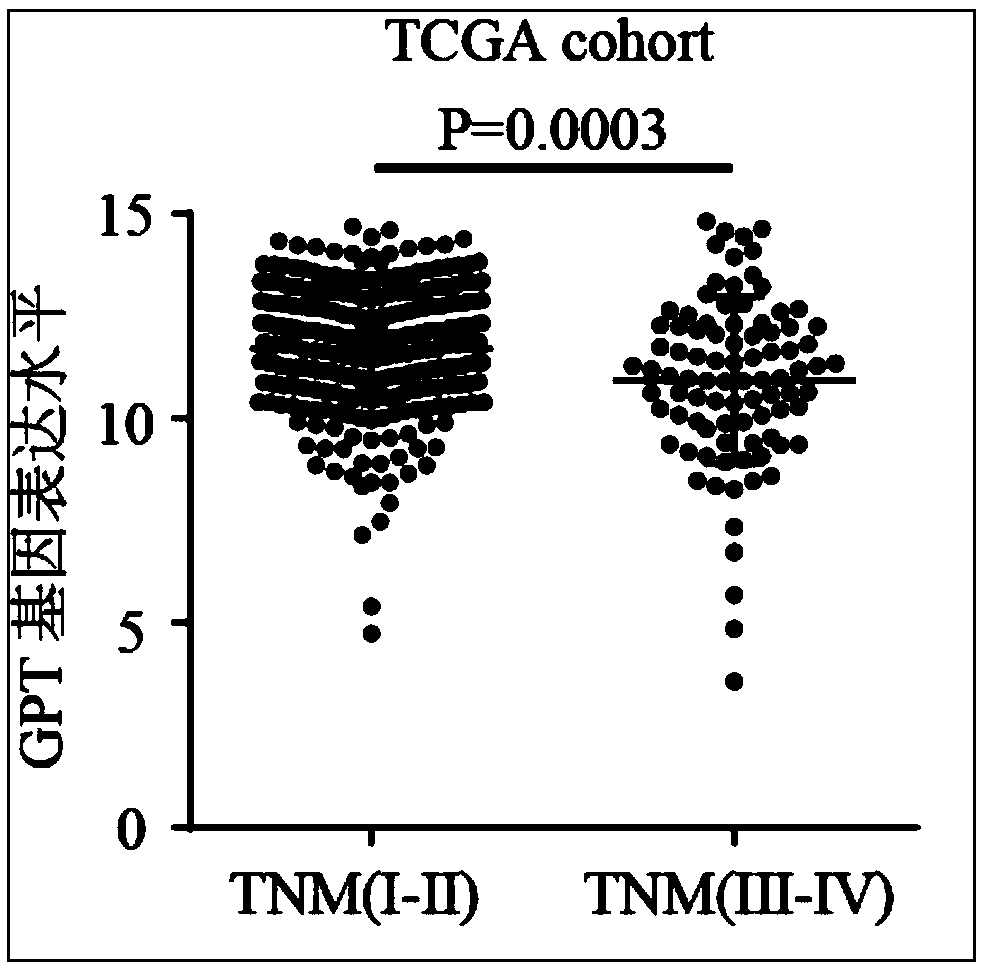
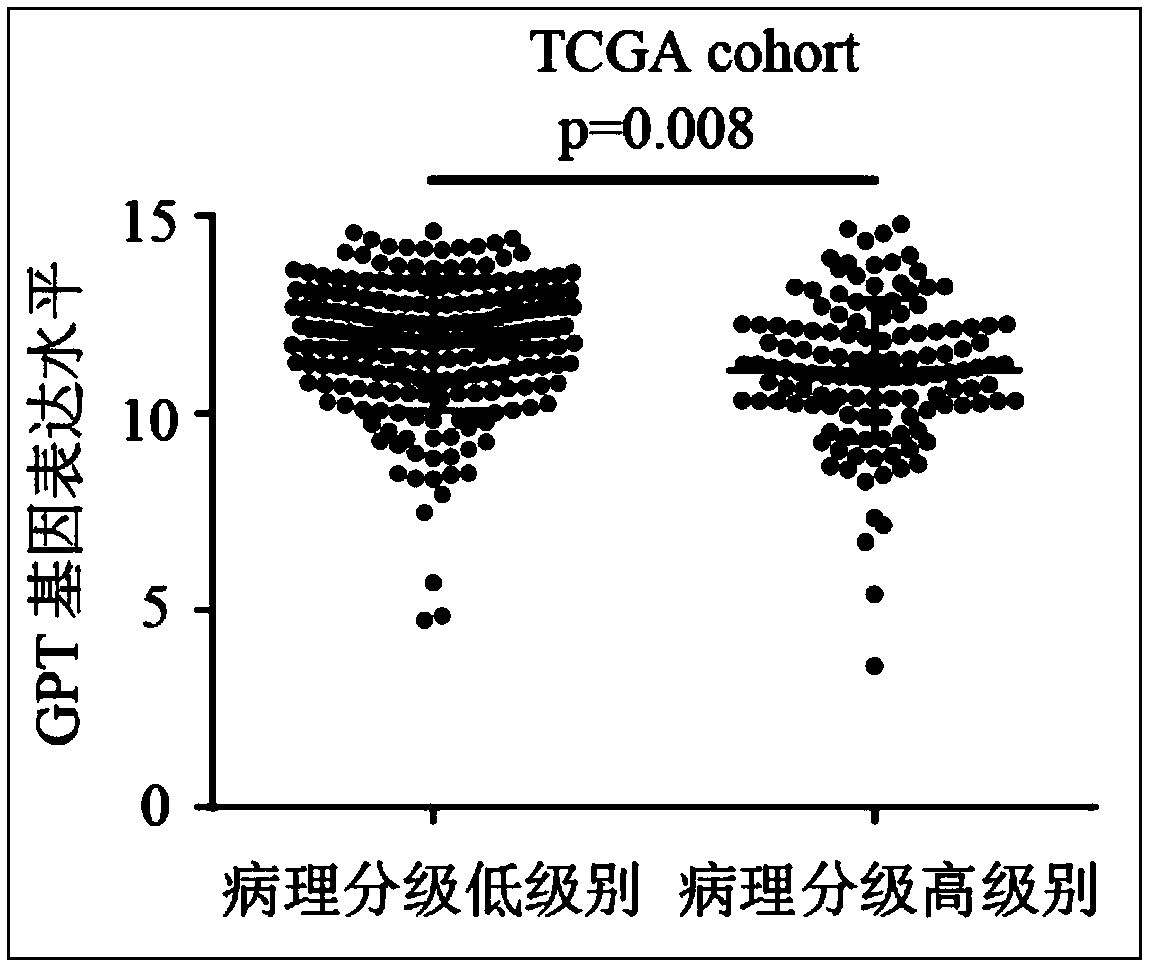
The invention relates to the technical field of biomedicines, discloses a human liver cancer marker and application thereof, and particularly relates to application of a GPT gene to diagnosis and treatment of hepatocellular carcinoma. By a gene chip data analysis and immunohistochemistry method, that the GPT gene is differentially expressed in a hepatocellular carcinoma tissue and a normal tissueis proved, and that expression of the GPT gene is closely related to clinical prognosis of a patient and the GPT gene can be used as an index for early diagnosing the hepatocellular carcinoma is confirmed. Through study on the expression of the GPT gene in liver cancer and a relationship between the GPT gene and the clinical prognosis, a new marker is provided for early diagnosis and prognostic judgment of the liver cancer so as to improve the early diagnosis rate of a liver cancer patient and improve the clinical prognosis of the patient.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
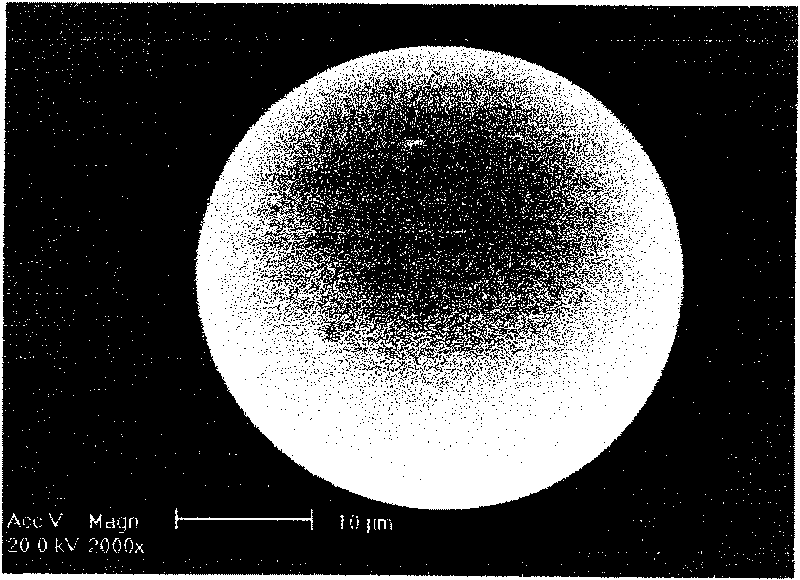
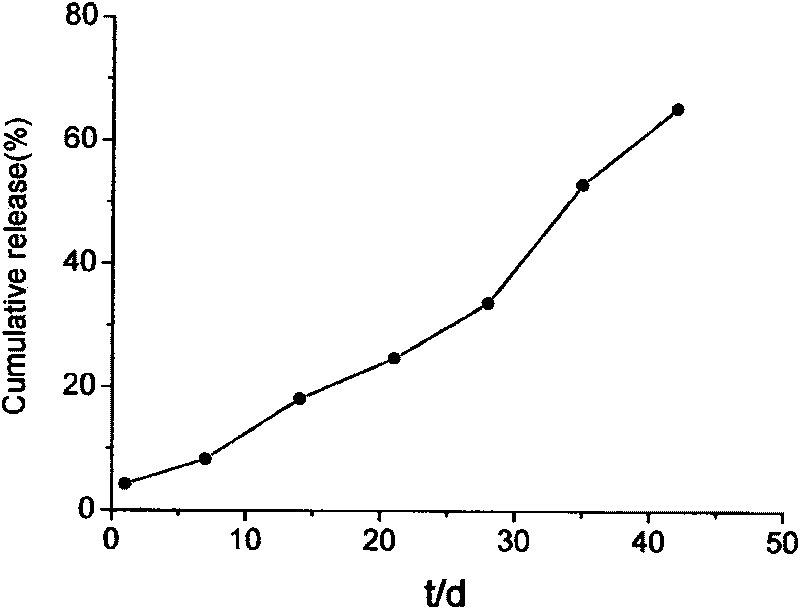
Method for preparing PLGA slow-release microsphere carrying docetaxel and application thereof in chemotherapy of mesenchyma stroma of tumors under ultrasonic mediation
InactiveCN101697963AImprove protectionComplete structureOrganic active ingredientsPharmaceutical non-active ingredientsSide effectDocetaxel
The invention discloses a method for preparing a PLGA slow-release microsphere carrying docetaxel and an application thereof in the chemotherapy of the mesenchyma stroma of tumors under ultrasonic mediation. The PLGA slow-release microsphere carrying the docetaxel is a medicine-carrying microsphere prepared from PLGA and the docetaxel by a single emulsification method, the molecular weight of the PLGA in the medicine-carrying microsphere is 5000-50000dal, the mole ratio of lactic acid to glycollic acid is 75:25-50:50, the feeding ratio of the PLGA to the docetaxel is 100 / 1-100 / 10, and the PLGA slow-release microsphere carrying the docetaxel is prepared by emulsification in an organic solvent. The slow-release microsphere preparation carrying the docetaxel is injected into the tumor tissues of nude mice with human liver cancer, beast cancer and ovarian cancer, and the necrosis situation of the tumors is checked by pathological histology; the result shows that the slow-release microsphere carrying the docetaxel can thoroughly ablate and inactivate the tumors, reduces the whole-body toxic or side effect of the medicine obviously and is a novel method for the chemotherapy of the mesenchyma stroma of the tumors under ultrasonic intervention with very good clinical application prospect.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS10512661B2Improvements in intestinal dysbiosisReduce intestinal permeabilityOrganic active ingredientsDigestive systemDiseaseTriacylglycerol VLDL
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
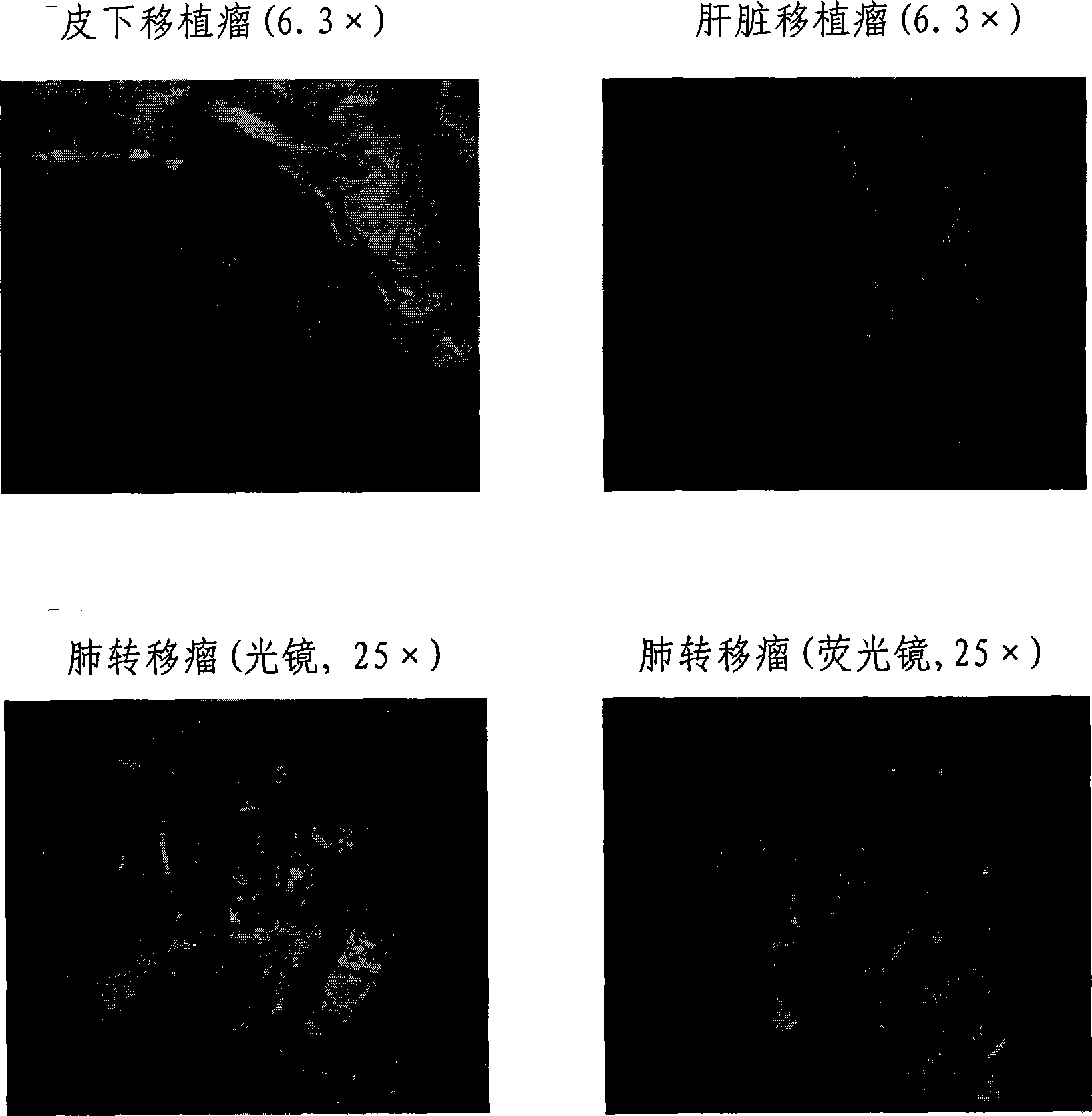
Installation method of fluorescent visual high-transfer human liver cancer nude mouse model
InactiveCN101380478AHard to quenchUniform fluorescence intensityTissue cultureFermentationAbnormal tissue growthTherapeutic effect
The invention belongs to the field of microorganism animal cell system, and relates to an establishment method of a fluorescence visualization human hepatoma nude mice model which can self emit high strength red or green fluorescences and has the metastatic ability. After obtaining a human high-metastatic fluorescence hepatoma cell line HCCLM3-R, HCCLM3-G, HCCLM6-R and HCCLM6-G of fluorescence genes with high chromosome conformable degree, high fluorescence intensity and stable expression by using the method of pseudotype slow virus infection, hepatoma cells expressed by the fluorescences is placed beneath nude mice skin to establish a fluorescence visualization high-metastatic human hepatoma nude mice subcutaneous tumor model or subcutaneous fluorescence expression tumor tissues is placed in nude mice hepar to establish a fluorescence visualization high-metastatic human hepatoma nude mice hepar primary tumor model. The got model can be used as a tracer of animal in vivo hepatoma cells, and can be used for molecular mechanism study of hepatoma recurrence and metastatic and prophase therapeutic effect discrimination for new anti-hepatoma therapy and new anti-hepatoma drugs, etc.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
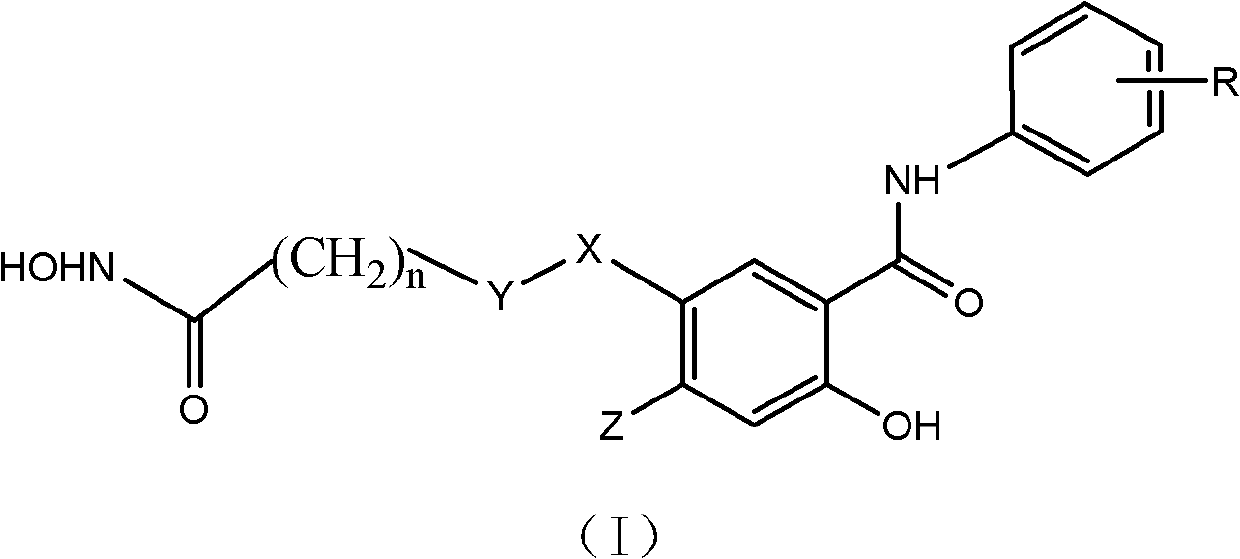
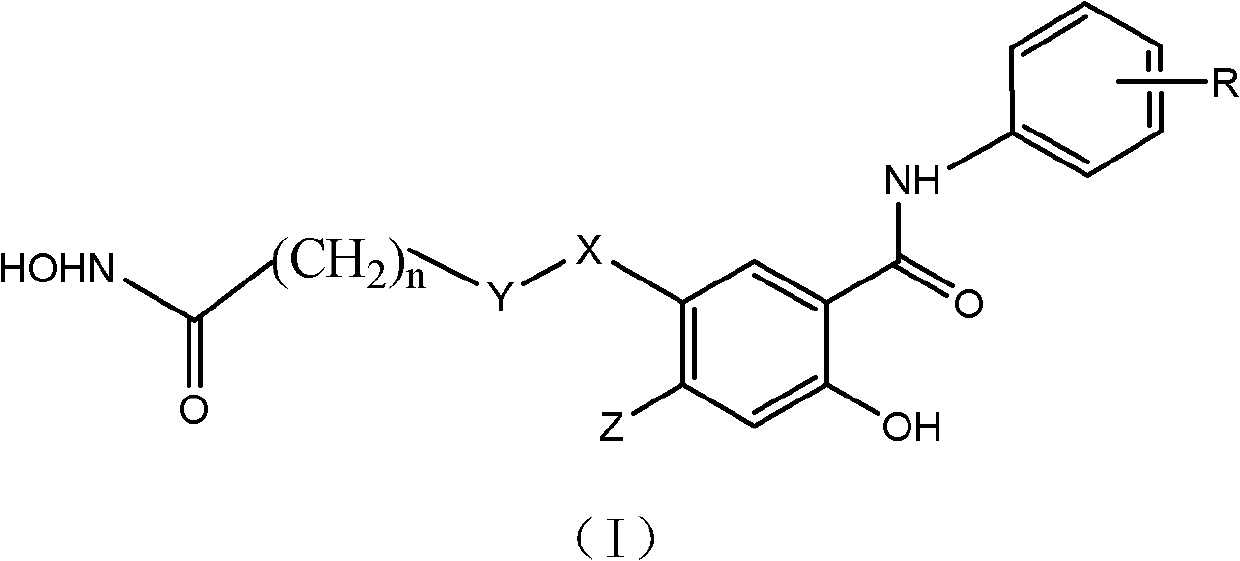
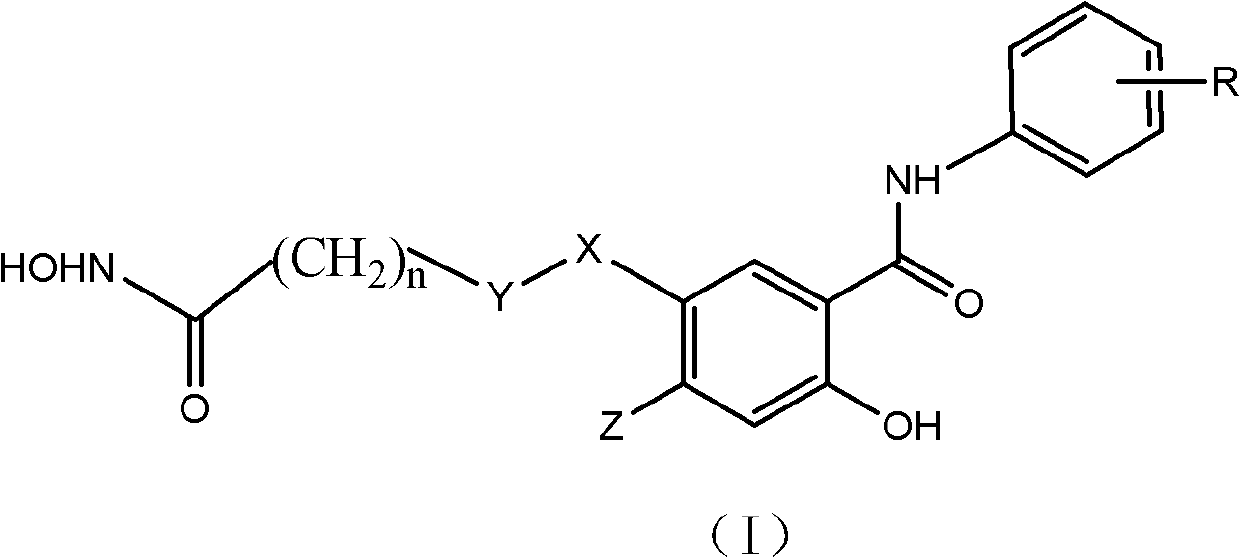
Salicylamide antitumor compound and its synthesis method and application
InactiveCN102276500ASimple structureThe synthesis method is simpleOrganic active ingredientsOrganic chemistryTrifluoromethylCarbonyl group
The invention discloses a class of compounds with antitumor activity. The structural formula is as follows: In the formula, R is one or two substituents of ethynyl, chlorine, fluorine, trifluoromethyl and 3-fluorobenzyloxy; X is O or NH; Y is CH2 or CO (carbonyl); Z is H or methoxy; n=5 or 6. The salicylic amide compounds in the structural formula are synthesized from 5-nitrosalicylic acid or 4-methoxysalicylic acid and aniline derivatives through amidation, reduction, and reaction with monoethyl pimelic acid chloride. . The compound has a novel structure and the synthesis method is easy to realize. The anti-tumor activity test showed that it has inhibitory effect on the growth of human liver cancer cells (HepG-2), human colon cancer (SW 480) and human prostate cancer cells (PC3), and the activity of most compounds is stronger than that of gefitinib. The use of preparing anti-tumor drug preparations.
Owner:XI AN JIAOTONG UNIV
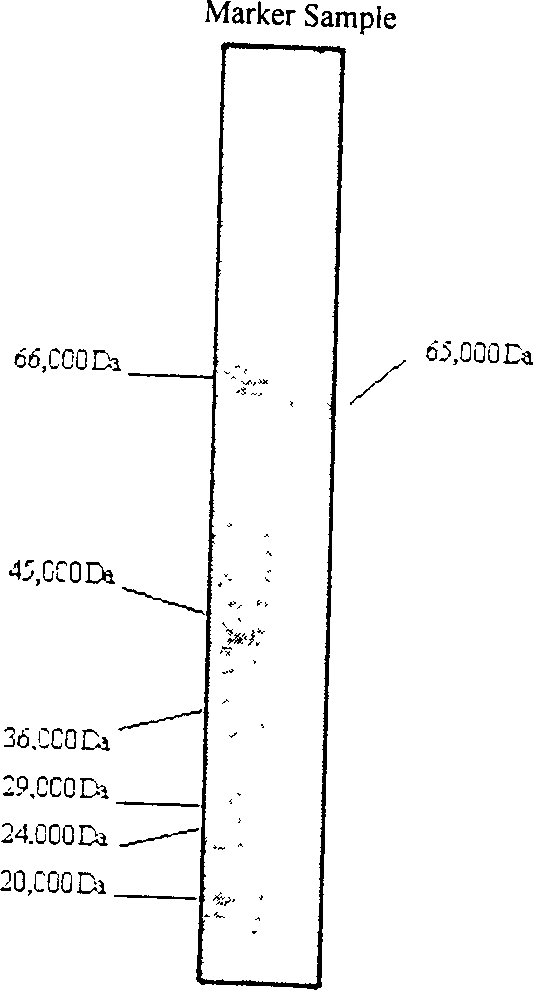
Protein extracted from perinereis aibuhitensis, its preparation method and use
InactiveCN1521188AHigh antibacterial activityPrevent proliferationAntibacterial agentsPeptide/protein ingredientsEscherichia coliPerinereis aibuhitensis
The present invention is one kind of protein extracted from Perinereis aibuhiteris and its preparation process and use. The protein is alkali protein with molecular weight 65000 Da and isoelectric point of 8.3. The protein preparing process includes raising Perinereis aibuhiteris in artificial sea water for 24-48 hr to make it become clean, electrically striking Perinereis aibuhiteris with 5-10 V DC to obtain body fluid, centrifugally filtering body fluid, reverse concentration, gel filtering, and chromatographic elution in anionic exchange column to collect active protein. The protein is used in preparing antibiotic medicine resisting escherichia coli, verdigris pseudomonad, staphylococcus aureus and arthrobacter and anticancer medicine for suppressing human liver cancer cell strain proliferation and producing alpha-fetoglobulin, and has high antibiotic activity and obvious anticancer effect.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
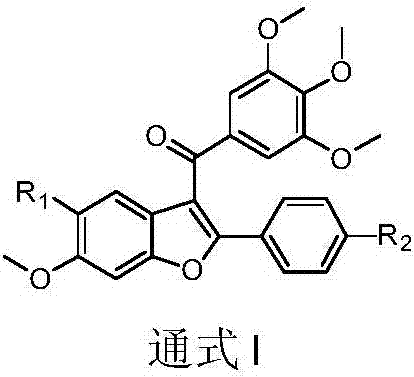
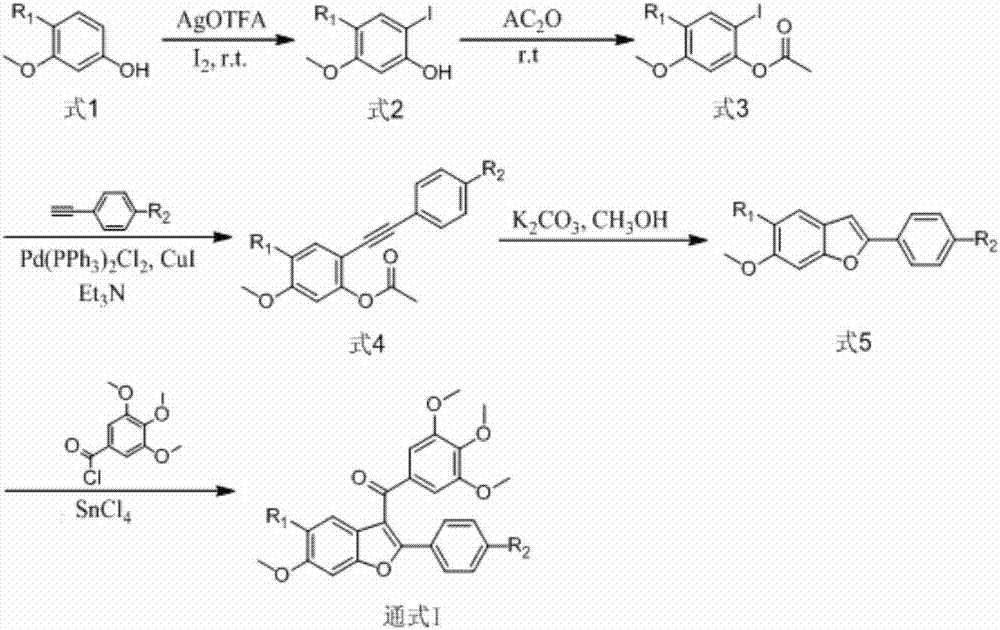
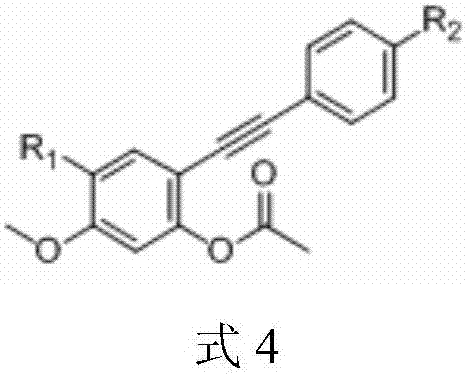
3-(3,4,5-trimethoxybenzoyl)-benzofuran microtubulin inhibitor as well as preparation method and use thereof
ActiveCN107163011AStrong inhibitory activityInhibition of polymerizationOrganic chemistryAntineoplastic agentsMedicineColchicine
The invention discloses a 3-(3,4,5-trimethoxybenzoyl)-benzofuran microtubulin inhibitor as well as a preparation method and use thereof. A 3-(3,4,5-trimethoxybenzoyl)-benzofuran compound in the invention has an action mechanism similar to colchicine and is capable of inhibiting polymerization of microtubulins. The 3-(3,4,5-trimethoxybenzoyl)-benzofuran compound has very strong proliferation inhibition activity to human lung cancer cells A549, human gastric cancer cells MGC-803, human liver cancer cells HepG2, human colon cancer cells HCT-116, human cervical cancer cells HeLa and human breast cancer cells MCF-7.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
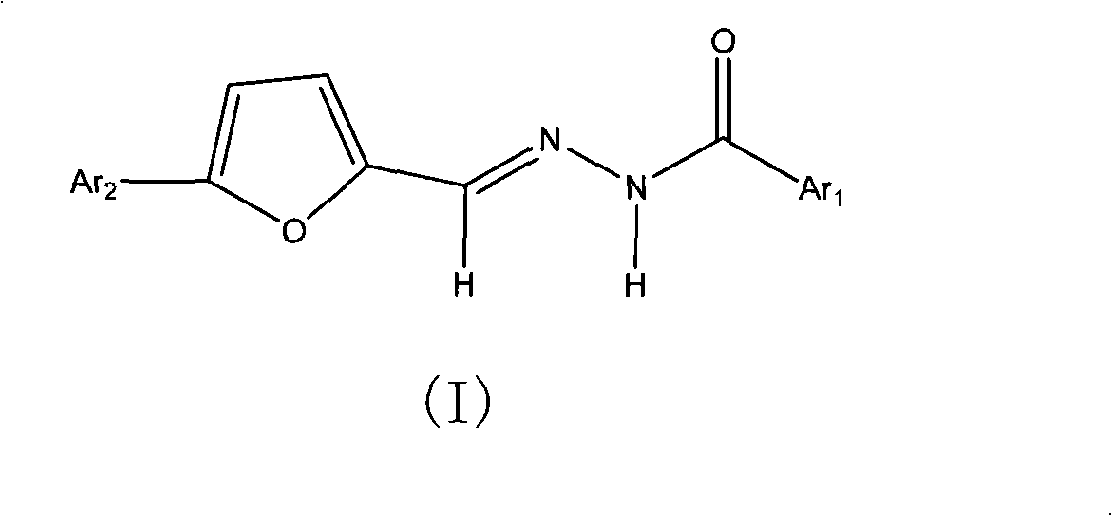
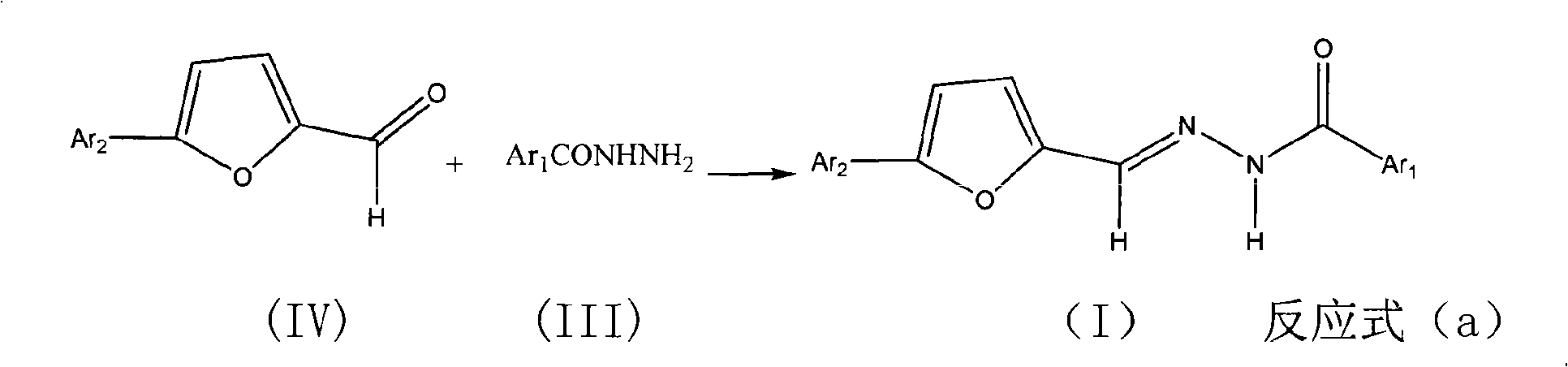
Benzoyl hydrazone compounds with antineoplastic activity
The invention relates to an N'-5-substitutent aryl-2-furan-N-substitutent benzoyl hydyne compound (I) with a novel structure. The compound is produced by reaction of substituted aryl formohydrazide and 5-substitent aryl furan-2-formaldehyde. The compound is found through research to have anti-tumor activities in which people are interested, particularly the activities for resisting stomach cancer, human liver cancer and nasopharynx cancer.
Owner:CHINA AGRI UNIV
A monoclonal antibody for serological diagnosis of liver cancer and its use
ActiveCN102276721AImmunoglobulins against animals/humansMicroorganism based processesAntigenImmunoconjugate
The invention relates to a monoclonal antibody for serological diagnosis of liver cancer and application thereof, and particularly provides an anti-human liver cancer specific monoclonal antibody 7C8. The monoclonal antibody has the characteristics of high stability and high specificity in combination with liver cancer antigen. The invention also provides 7C8 immunoglobulin and a fragment as well as an immunological conjugate thereof and a medicinal composition containing the immunoglobulin, the fragment or the immunological conjugate. The invention also provides a kit for detecting liver cancer.
Owner:SHANGHAI INST OF ONCOLOGY
Mice transplanted with human hepatocytes
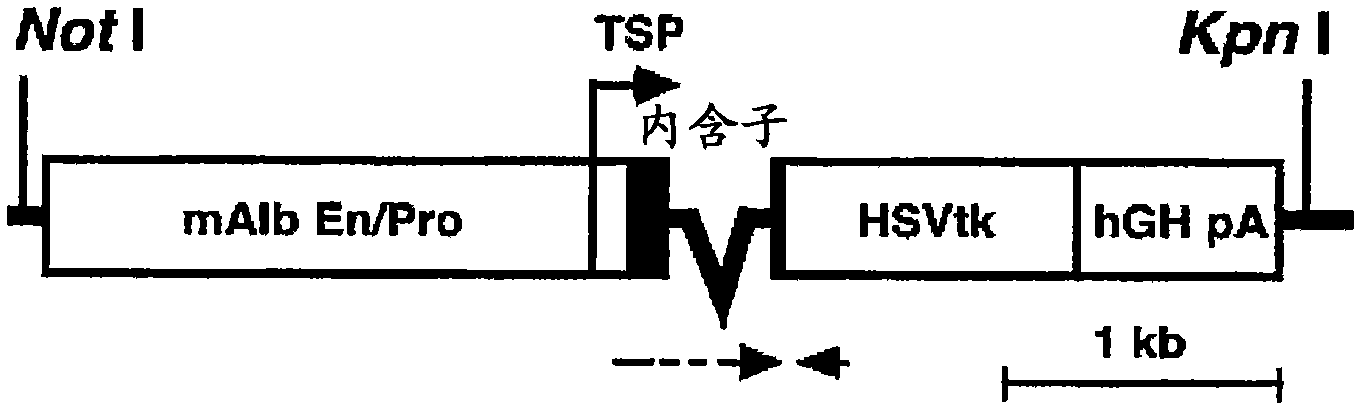
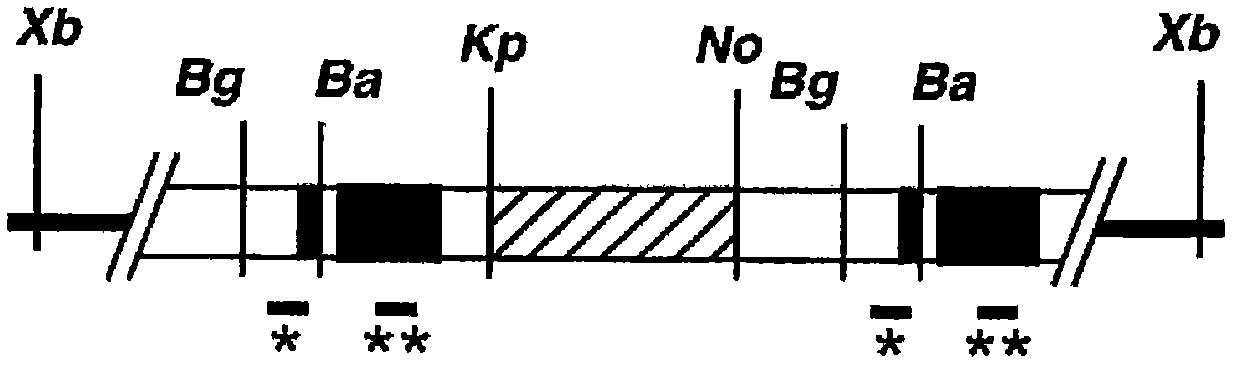
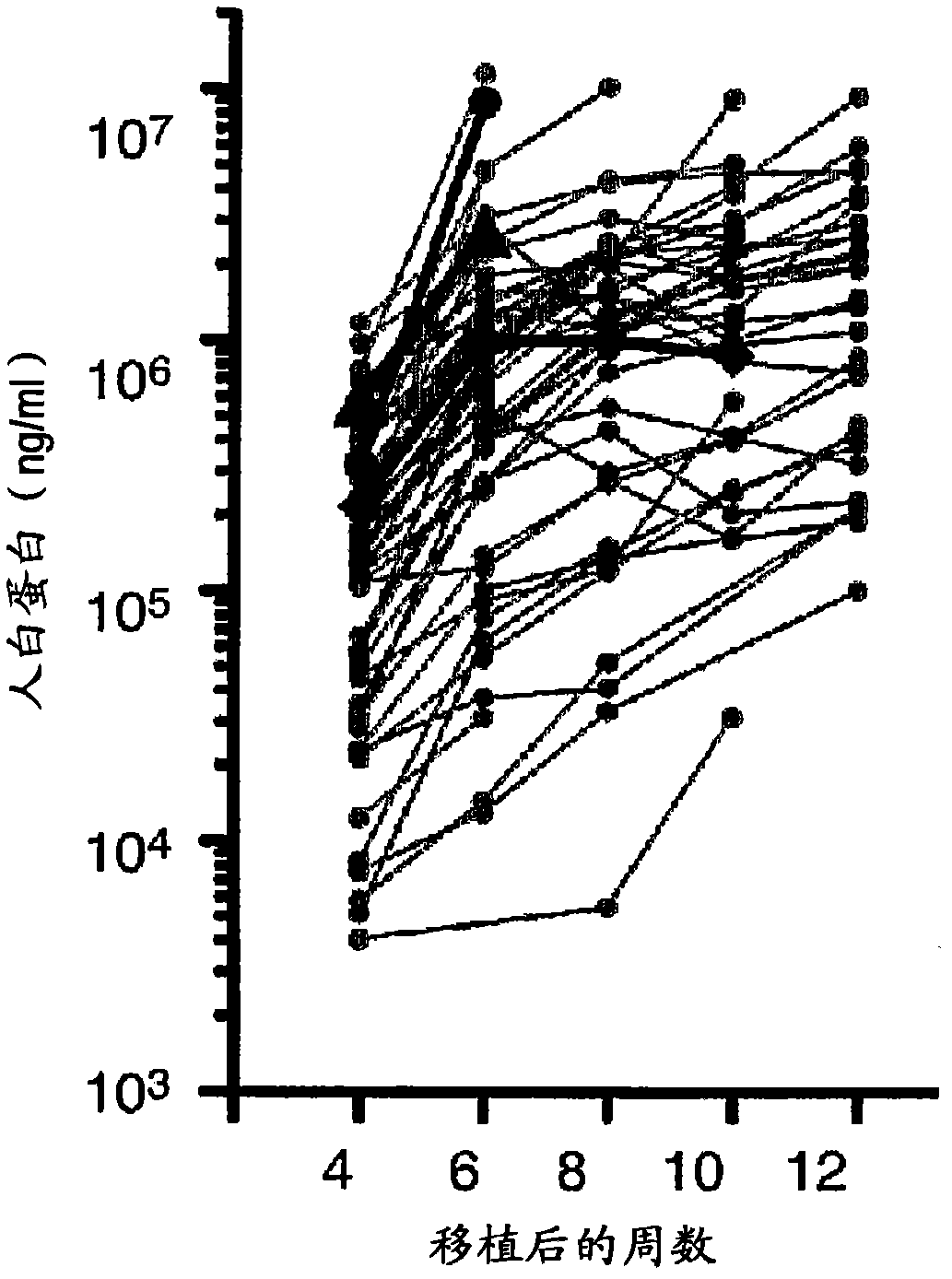
Disclosed is a mouse having human hepatocytes transplanted therein. Specifically disclosed is a mouse having human hepatocytes transplanted therein. In the mouse, a foreign thymidine kinase gene or an urokinase-type plasminogen activator gene is retained so that the gene can be expressed specifically in the liver of the mouse, and hepatocytes of the mouse are substituted by human hepatocytes.
Owner:CENT INST FOR EXPERIMENTAL ANIMALS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com