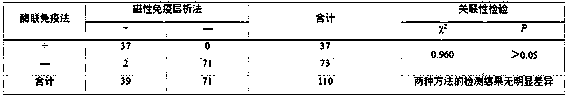
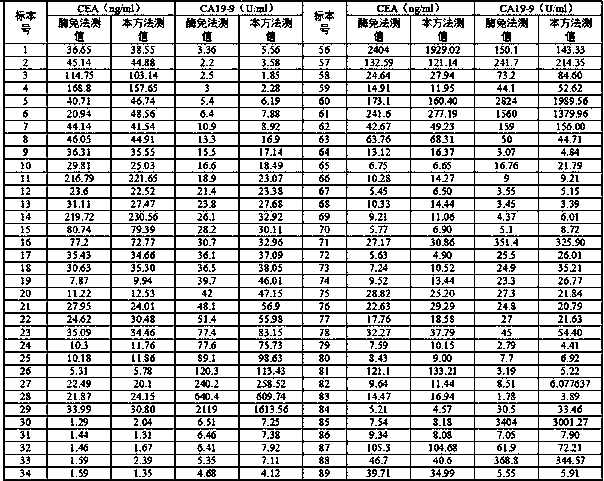
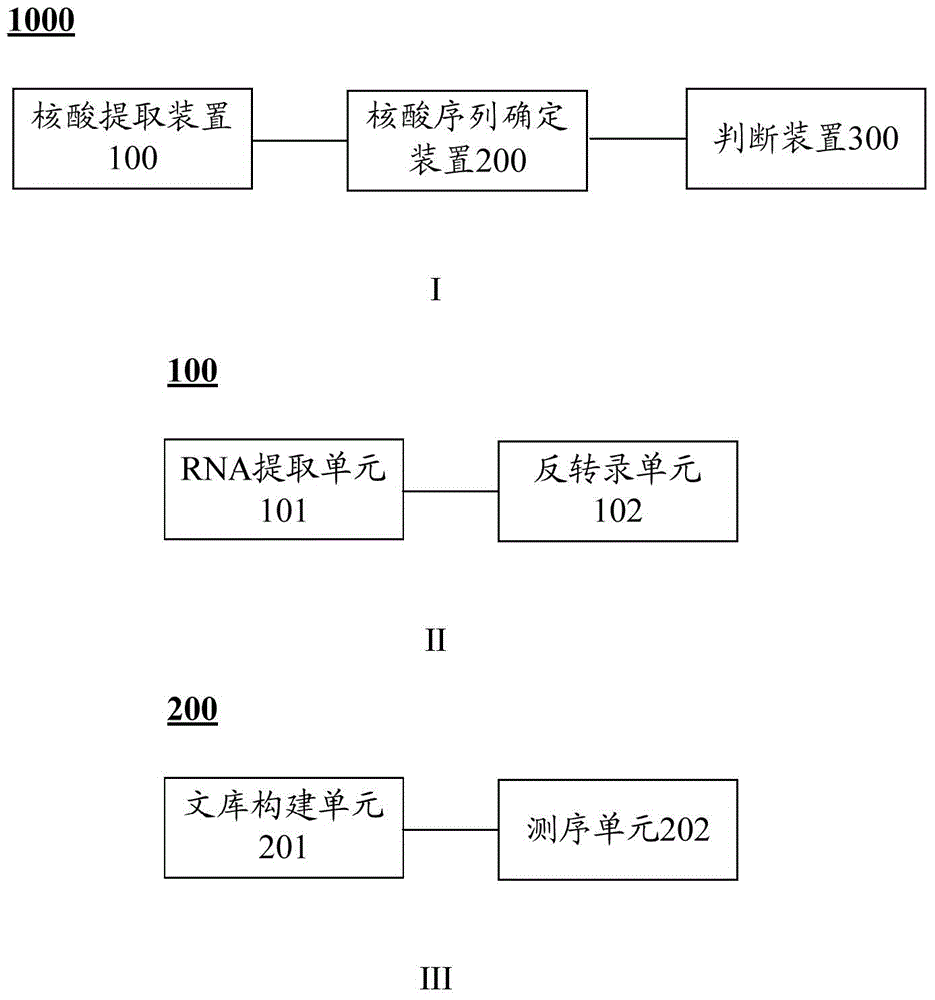
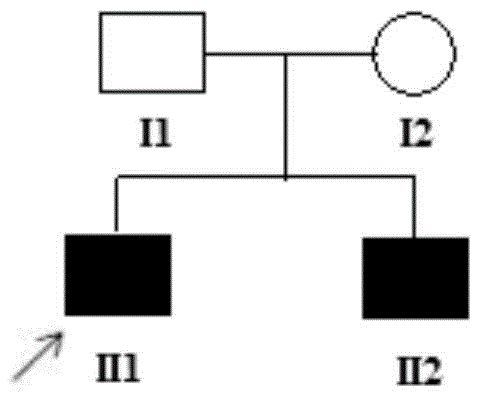
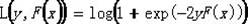Patents
Literature
60results about How to "Improve early diagnosis rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
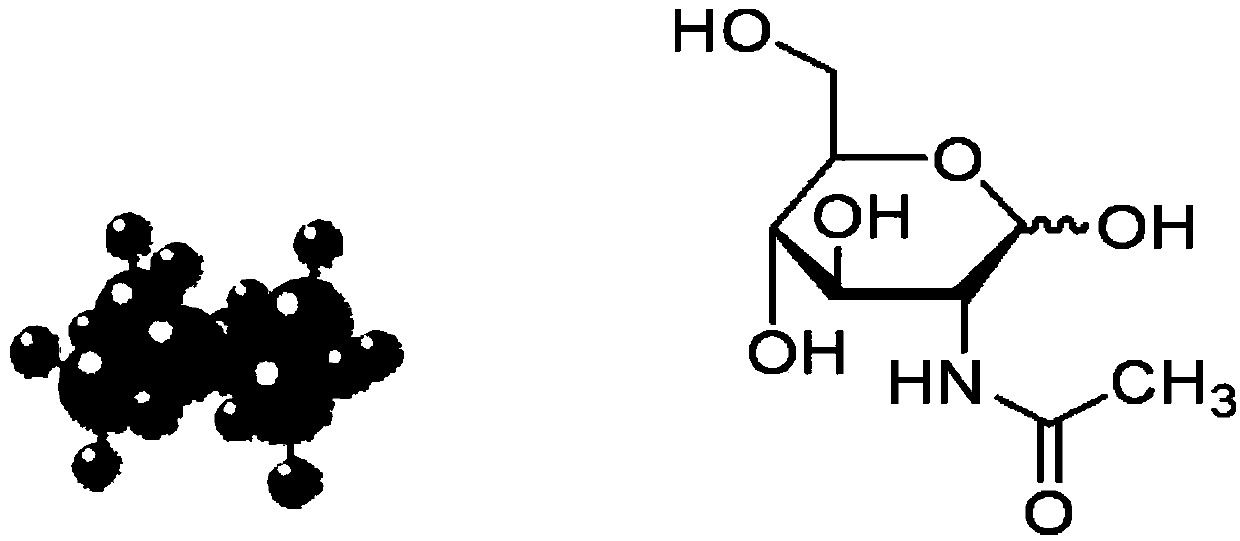
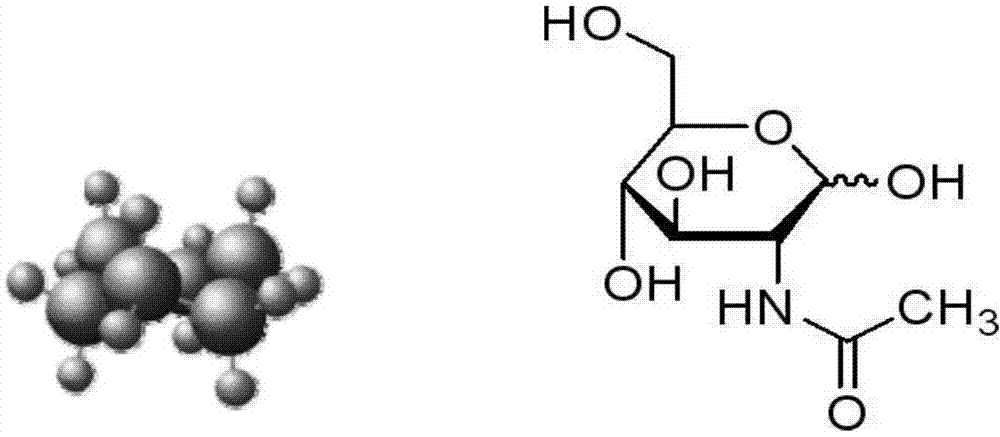
Application of N-acetylglucosamine in preparing kit for detecting tumor
InactiveCN104198707AImprove early diagnosis rateImprove the detection rateMaterial analysisDNA/RNA fragmentationNormal peopleMedicine
The invention discloses application of N-acetylglucosamine in preparing a kit for detecting tumor. According to the technical scheme disclosed by the invention, the detection rate of the tumor is greater than 90%, and the tumor specificity is greater than 95%. The early-stage diagnostic rate of the tumor is greatly increased. The kit is applicable to early-stage general survey on tumor of normal people.
Owner:范飞舟 +1
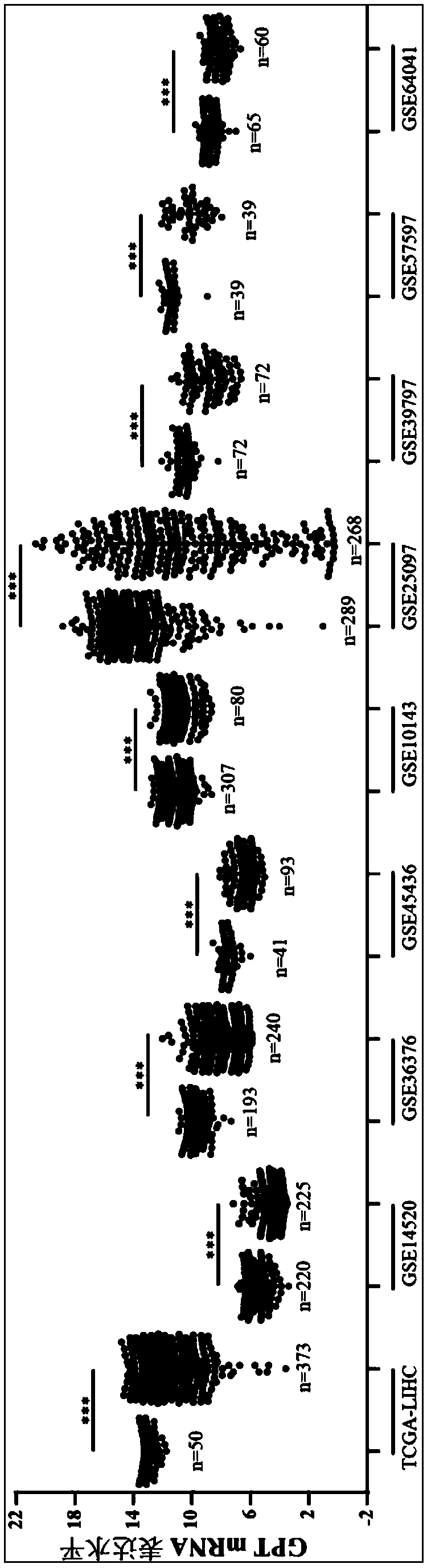
Human liver cancer marker and application thereof
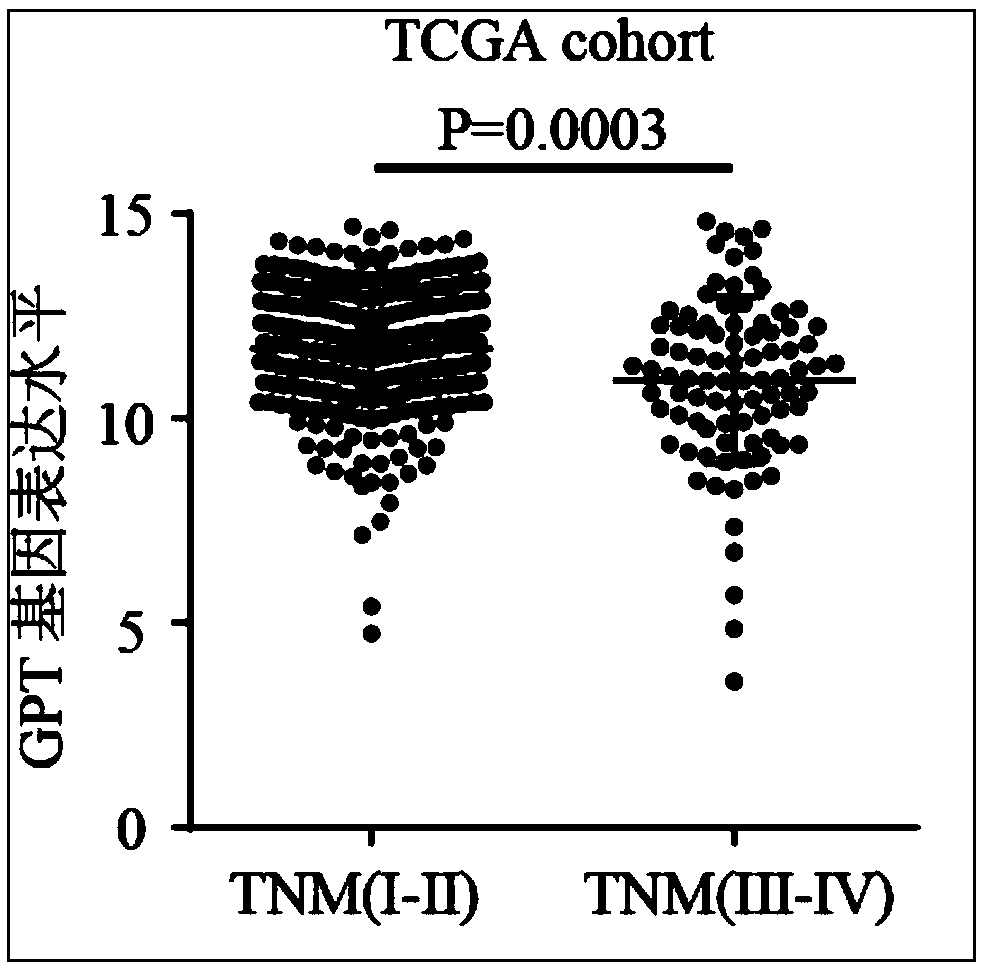
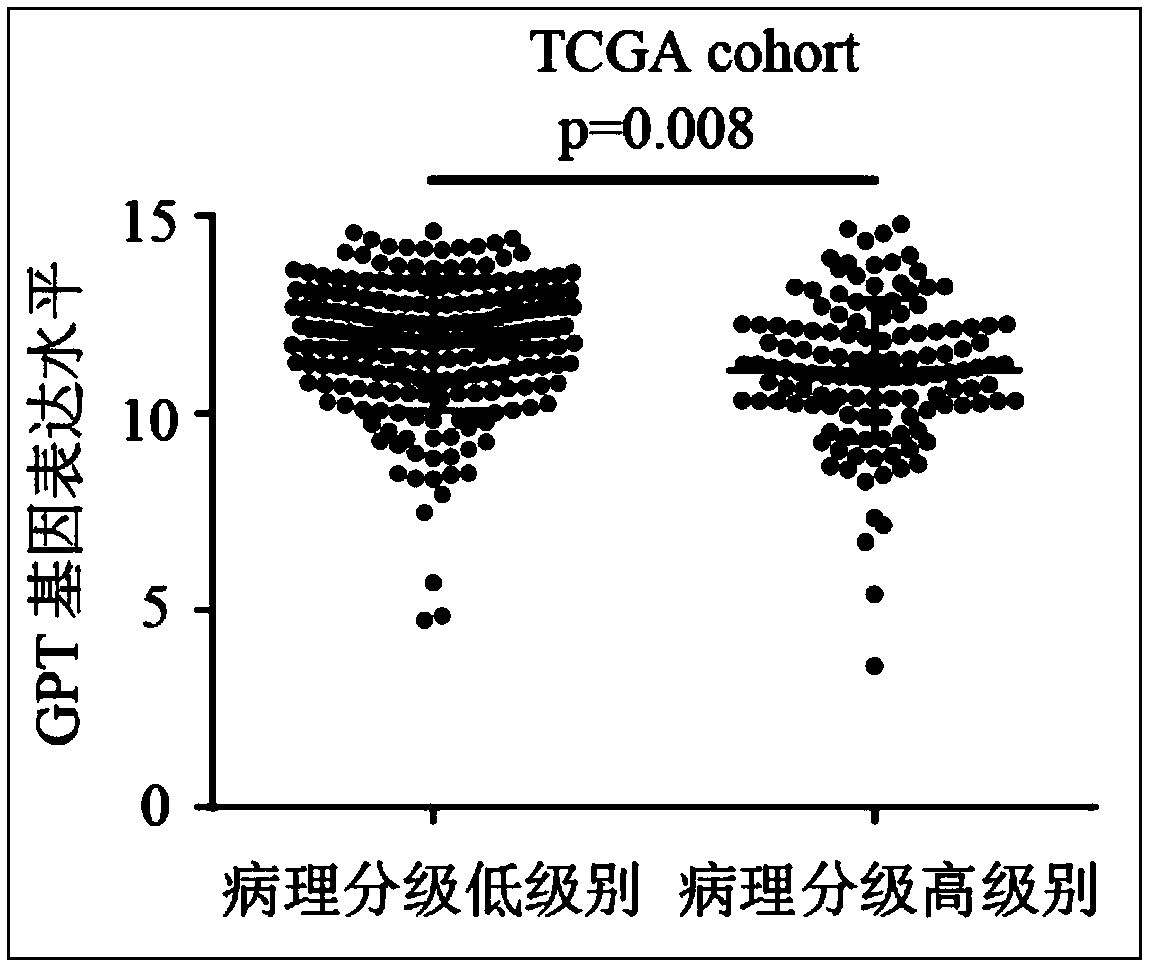
InactiveCN108315413AHigh expressionImprove early diagnosis rateMicrobiological testing/measurementMaterial analysisDiagnosis earlyClinical prognosis
The invention relates to the technical field of biomedicines, discloses a human liver cancer marker and application thereof, and particularly relates to application of a GPT gene to diagnosis and treatment of hepatocellular carcinoma. By a gene chip data analysis and immunohistochemistry method, that the GPT gene is differentially expressed in a hepatocellular carcinoma tissue and a normal tissueis proved, and that expression of the GPT gene is closely related to clinical prognosis of a patient and the GPT gene can be used as an index for early diagnosing the hepatocellular carcinoma is confirmed. Through study on the expression of the GPT gene in liver cancer and a relationship between the GPT gene and the clinical prognosis, a new marker is provided for early diagnosis and prognostic judgment of the liver cancer so as to improve the early diagnosis rate of a liver cancer patient and improve the clinical prognosis of the patient.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
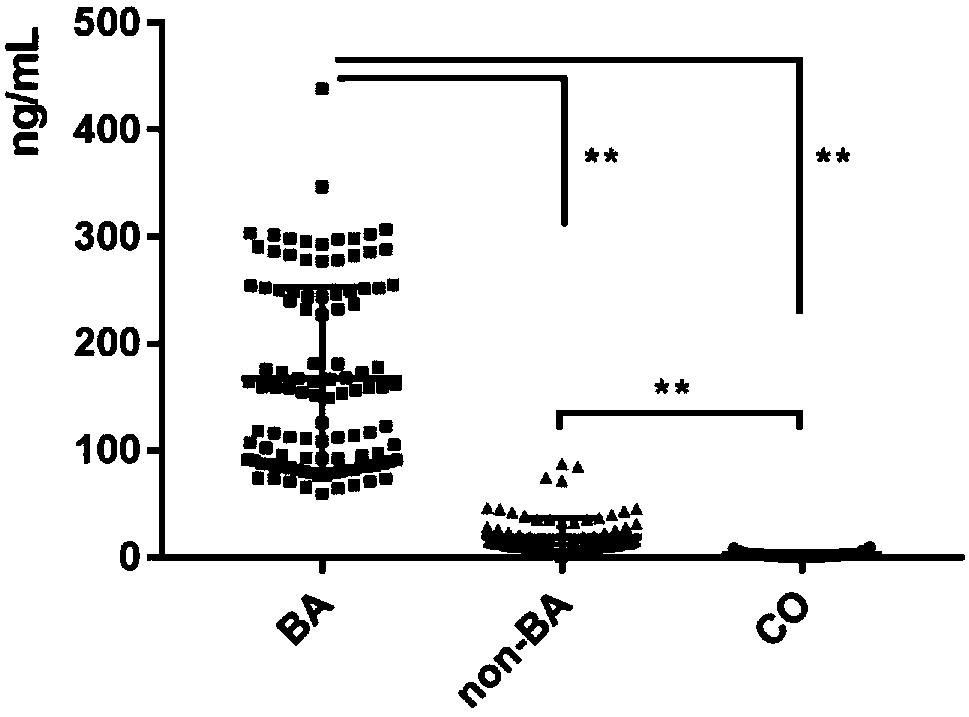
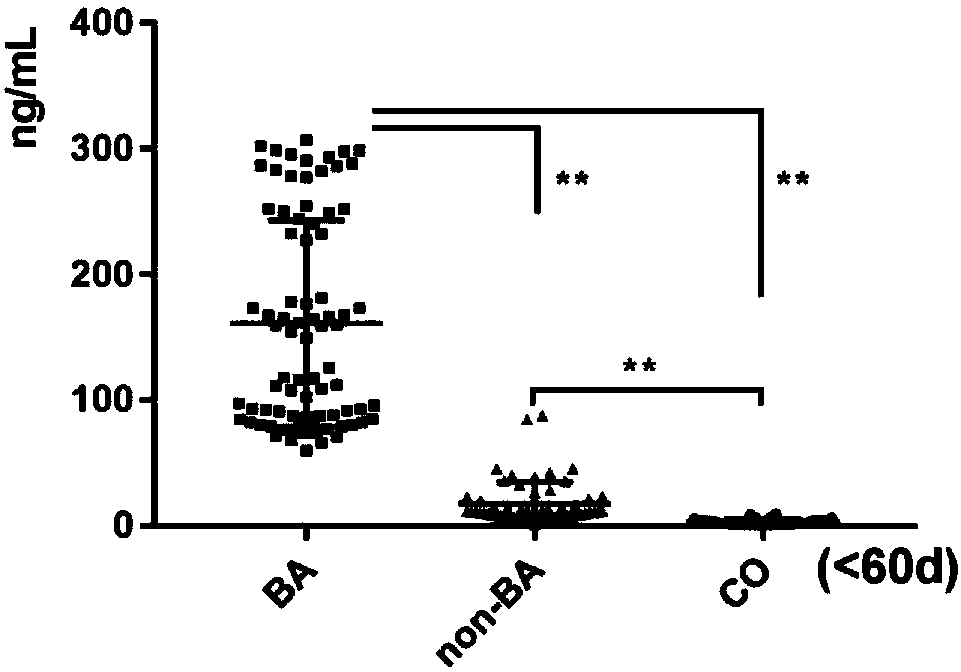
Serum marker MMP-7-based biliary atresia diagnosis kit
InactiveCN108267585AEasy to operateAid in early diagnosisDisease diagnosisPositive controlPRIMARY BILIARY ATRESIA
The invention relates to a serum marker MMP-7-based biliary atresia diagnosis kit. The diagnosis kit comprises an anti-human MMP-7 monoclonal antibody coated ELISA plate, a negative control solution,a positive control solution, an enzyme labeling reagent, an enzyme substrate solution, a blocking solution, a sample diluent, a washing solution and a stopping solution. The diagnosis kit is a new sensitive, safe, reliable and easily-operated commercial kit. Quantitative detection of the level of MMP-7 in human serum is helpful for early diagnosis of BA; the BA diagnosis sensitivity of the serum biomarker protein MMP-7 is 100%, and the BA diagnosis specificity is 95.6%, so the kit has the characteristics of high specificity and high sensitivity; and the kit improves the early diagnosis rate ofBA, reduces misdiagnosis, and improves the self liver survival rate of the BA.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Method for detecting mammaglobin and kit therefor
InactiveCN1766634AShorten test timeReliable test resultsMaterial analysisAgglutinationMammary gland structure
The invention relates to method and agent box for detecting mammary gland globulin in the field of biology chemistry. The latex fixation leptospiral method comprises the steps of: a) mixing the mammary gland globulin antibody marked emulsion particle with the tested sample, b) quoting the test result by weather it has agglutination reaction, wherein the mammary gland globulin antibody marked emulsion particle is the emulsion particle which uses mammary gland globulin antibody R028 or R048 marked emulsion particle with the dilemma 2-10um; preserving the marked emulsion particle inside the HEPES buffer solution with pH 8.2. It also discloses a method for using the anti-mammary gland globulin monoclonal antibody and skin factor mark anti-mammary gland globulin monoclonal antibody to quantity test the mammary gland globulin by double clamp heart enzyme immune adsorption method. It also discloses a RT-PCR test mammary gland globulin expressing method and provides the agent box used to test the mammary gland globulin.
Owner:初培国
Kit for detecting tumors and special substance for identifying N-acetylglucosamine of kit
InactiveCN104267185AImprove early diagnosis rateImprove the detection rateMaterial analysisDNA/RNA fragmentationNormal peopleN-Acetylglucosamine
The invention discloses a kit for detecting tumors. The kit contains a substance for detecting the tumors. The substance for detecting the tumors is a substance which can be specifically combined with N-acetylglucosamine. By adopting the substance for detecting the tumors, the detection rate for a plurality of tumors is more than 90% and the tumor specificity is more than 95%. Therefore, the early-stage diagnostic rate of the tumors is greatly improved. The kit for detecting the tumors and the substance for detecting the tumors are suitable for early-stage general investigation of the tumors in normal people.
Owner:范飞舟
Parkinson's disease finger tapping action recognition method and system, storage medium and terminal
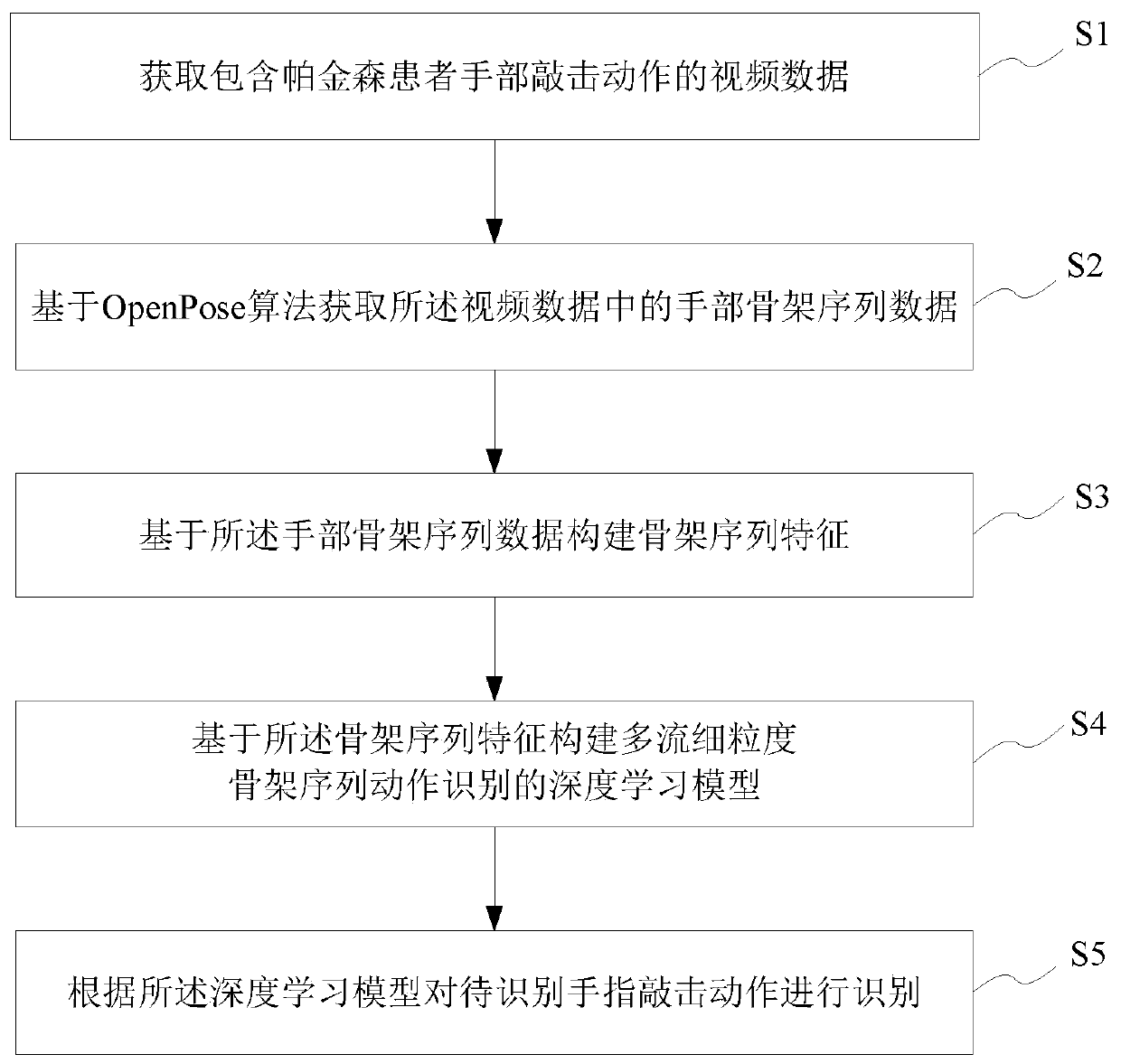
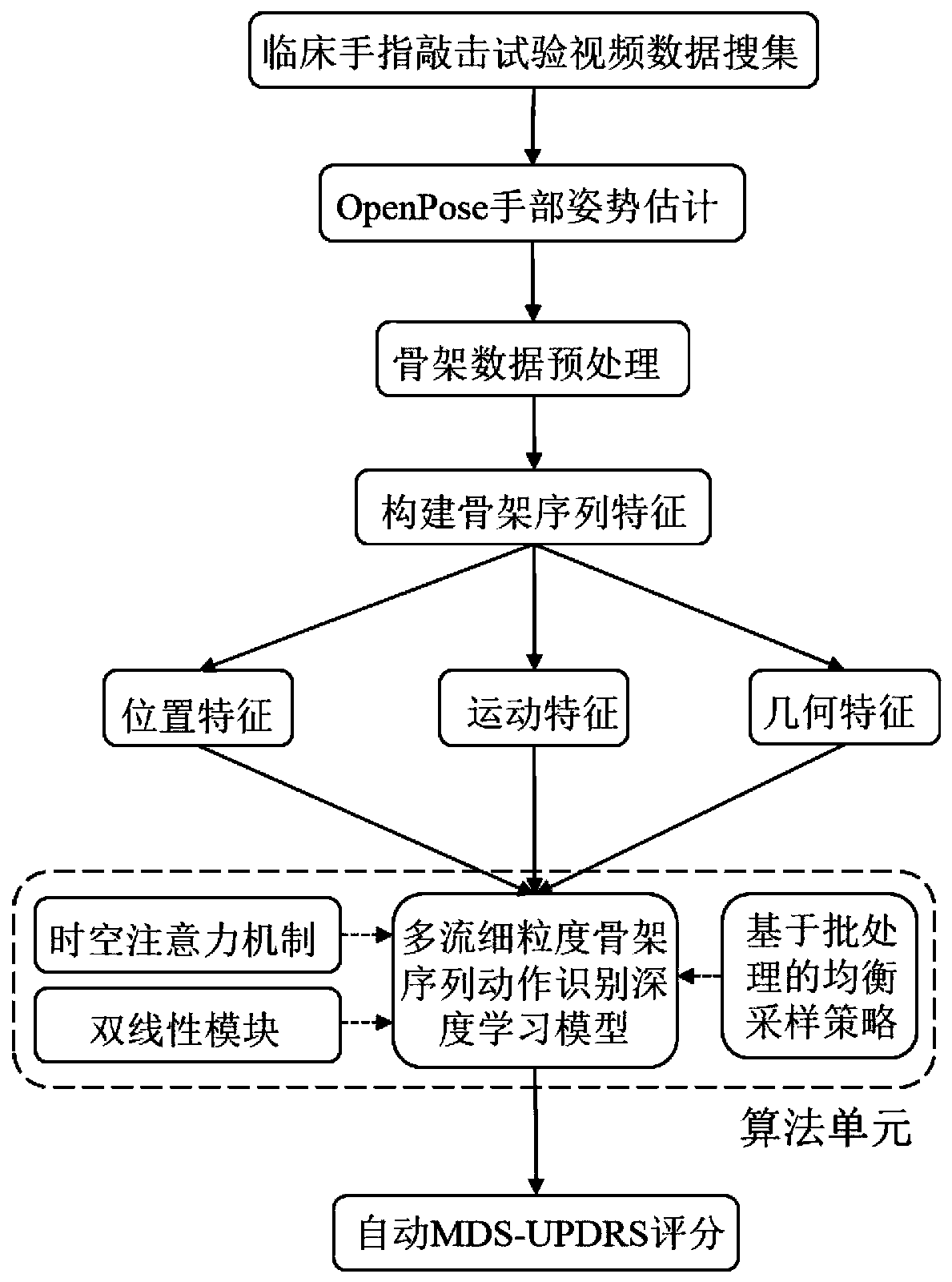
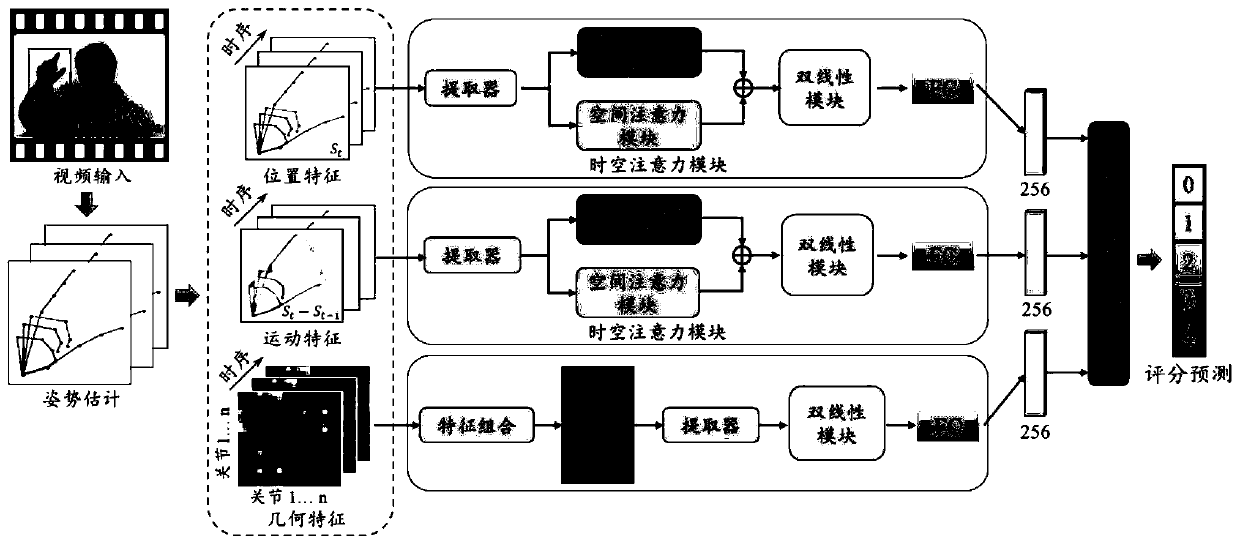
PendingCN111274998AImprove accuracy and robustnessImprove fine-grained classification capabilitiesMedical automated diagnosisCharacter and pattern recognitionSequencing dataNeuroscience
The invention provides a Parkinson's disease finger tapping action recognition method and system, a storage medium and a terminal. The Parkinson's disease finger tapping action recognition method comprises the following steps: acquiring video data including hand tapping actions of a Parkinson's disease patient; obtaining hand skeleton sequence data in the video data based on an OpenPose algorithm;constructing skeleton sequence features based on the hand skeleton sequence data; constructing a deep learning model for multi-stream fine-grained skeleton sequence action recognition based on the skeleton sequence features; and according to the deep learning model, identifying a to-be-identified finger tapping action. According to the Parkinson's disease finger tapping action recognition methodand system, the storage medium and the terminal, recognition of the Parkinson's disease finger tapping action is achieved based on the hand posture estimation algorithm and the deep learning algorithm, the accuracy is high, and the practicability is high.
Owner:SHANGHAI JIAO TONG UNIV
Sputum exfoliated cell preservation liquid and preparation method thereof
InactiveCN107258765AReduce processing timeAvoid damagePreparing sample for investigationDead animal preservationExfoliative cytologyPolyethylene glycol
The invention provides a sputum exfoliated cell preservation liquid with capacity of effectively improving a sputum specimen retention method and a slide preparation method as well as a preparation method of the sputum exfoliated cell preservation liquid. The sputum exfoliated cell preservation liquid is mainly prepared from polyethylene glycol-400 as a cell protecting agent, dithiothreitol as a mucus decomposer, ethyl alcohol as a cell stabilizer and the like. With the application of the sputum exfoliated cell preservation liquid, sputum can be preserved once spit, so that the sputum retention method is regulated, sputum collection time and storage time are prolonged, and cells in the sputum are prevented from autolysis and denaturation; meanwhile, the sputum treated with the preservation liquid can be applied to preparation of slides from liquid based thin-layer cells, therefore, the collection quantity of the cells is increased, the slide preparation effect is improved, and positive rate of sputum exfoliated cell detection is effectively increased. After application of the technology, sputum exfoliative cytologic examination can be simpler, more convenient, easier, safer and more economical and can be more easily popularized and used, and the sputum exfoliated cell preservation liquid and the preparation method thereof can be one of main methods for early diagnosis and screening of lung cancer, thereby effectively increasing early diagnosis rate and recovery rate of lung cancer and bringing benefits to humans.
Owner:杨珍杰
Magnetic immunochromatographic test strip capable of quantitatively detecting CEA and CA19-9 in blood simultaneously and preparation method
InactiveCN108020666ARealize quantitative detection of single servingHigh precisionMaterial analysisMedicineQuality control
The invention discloses a magnetic immunochromatographic test strip capable of quantitatively detecting CEA and CA19-9 in blood simultaneously and a preparation method. The test strip is prepared by successively binding a sample pad, a combining pad, a nitrocellulose membrane and water absorbing paper to a bottom plate of the test strip and 2mm of adjacent parts are alternately overlaid one another, wherein the nitrocellulose membrane is pre-coated with a detection line of a CEA antibody and a CA19-9 antibody and a quality control line of a rabbit antimouse IgG antibody. Compared with existingtumor marker detection technologies, the magnetic immunochromatographic technology is creatively introduced into combined quantitative detection of CEA and CA19-9 to achieve microscale quantitative analysis of CEA and CA19-9, so that the detecting sensitivity is improved greatly; and based on double positive CEA and CA19-9, the occurrence rate and transfer rate of tumor can be further judged to improve early stage diagnostic rate of occurrence and transfer of colon cancer to achieve the purpose of immediate detection and quick diagnosis, and the test strip is simple to operate and low in cost, and can be widely popularized.
Owner:FUZHOU UNIV
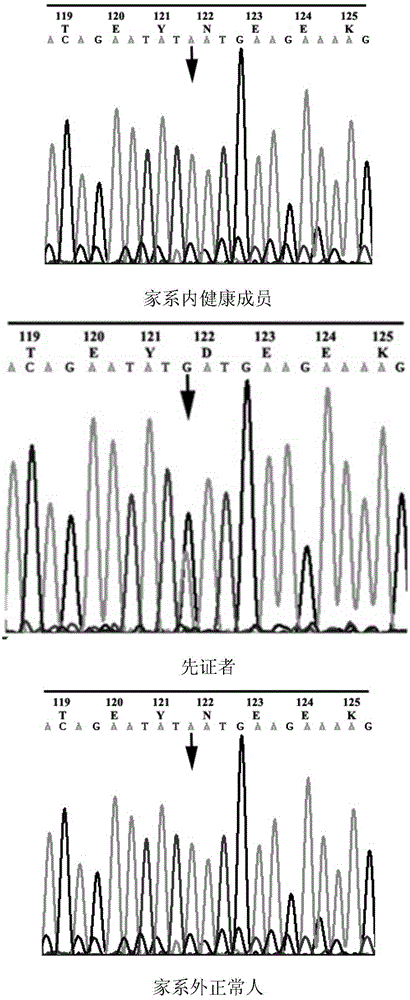
PTPRQ gene mutant and application thereof
ActiveCN105838720AImprove early diagnosis rateBioreactor/fermenter combinationsBiological substance pretreatmentsPTPRQ geneMutant
The invention discloses a PTPRQ gene mutant and an application thereof, and specifically relates to an isolated PTPRQ-mutant-coding nucleic acid, an isolated polypeptide, and a method, system, and kit for screening a biological sample susceptible to non-syndromic autosomal-recessive hearing loss. Compared with SEQ ID NO:1, the isolated PTPRQ-mutant-coding nucleic acid has c.3125A>G mutation or c.5981A>G mutation. Whether a new mutant exists in the biological sample is detected, and the fact that if the biological sample is susceptible to non-syndromic autosomal-recessive hearing loss can be effectively detected.
Owner:GENERAL HOSPITAL OF PLA +1
Diagnosis chip of recessive pathogenic genetic genes of Parkinson disease
ActiveCN103667500AReliable resultsImprove early diagnosis rateNucleotide librariesMicrobiological testing/measurementGenetic diagnosisGenomic clone
A diagnosis chip of recessive pathogenic genetic genes of a Parkinson disease has 96 mutational site sequences, namely 23 Parkin, 19 PINK1, 6 DJ-1, 14 ATP13A2, 25 PLA2G6 and 9 FBXO7. The chip comprises the following test steps of: fabricating an SUD plate, resuspending the SUD plate, fabricating an ASE plate, adding MEL, performing preliminary work on PCR (Polymerase Chain Reaction), combining PCR products, preparing a pre-chip sample, performing chip hybridization, cleaning and scanning the chip, and reading and analyzing data. The diagnosis chip of the recessive pathogenic genetic genes of the Parkinson disease is low in cost, and can screen the recessive pathogenic genetic genes of the Parkinson disease conveniently and rapidly.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Molecular marker miR-452 for early diagnosing acute kidney injury caused by septicopyemia, kit and application thereof
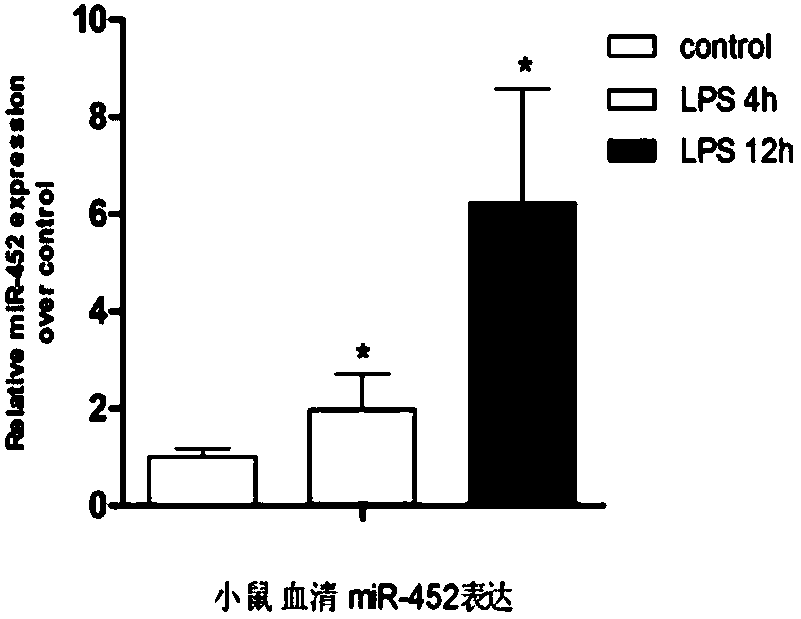
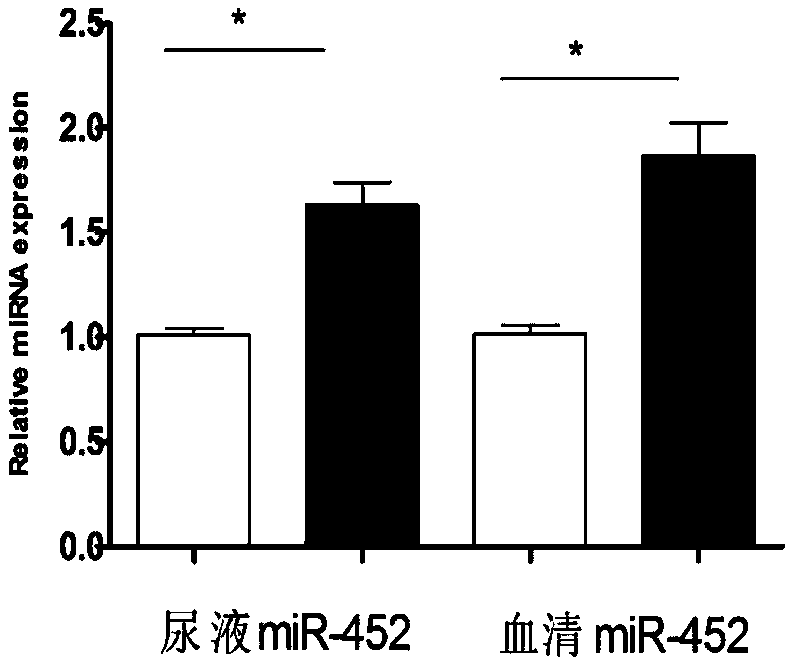
ActiveCN107699617AImprove early diagnosis rateImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationBacteriuriaDiagnosis early
The invention discloses a molecular marker miR-452 for early diagnosing acute kidney injury caused by septicopyemia, a kit and an application thereof. The molecular marker is used for early diagnosingof acute kidney injury caused by septicopyemia. In the body of a septicopyemia patient, the blood and urine miR-452 expressions are both up-regulated and both have certain relevance with serum creatinine but the relevance in urine is better, so that miR-452 expression up-regulation is possibly related to the renal injury. A diagnostic test shows that pure screening for urine miR-452 molecule is higher in sensitivity for diagnosing septicopyemia AKI but the combined screening for blood and urine miR-452 is capable of promoting the specificity of diagnosis. The result of the invention proves that miR-452 can be used as a biomarker for diagnosing septicopyemia AKI and has the advantage of early diagnosis and higher sensitivity and specificity.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
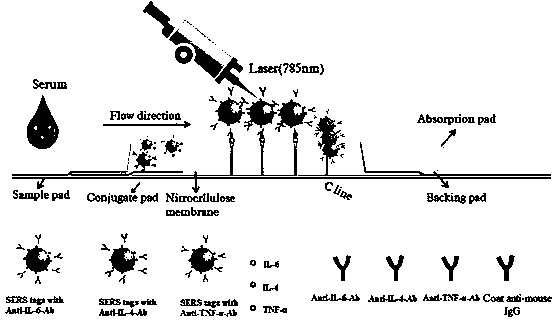
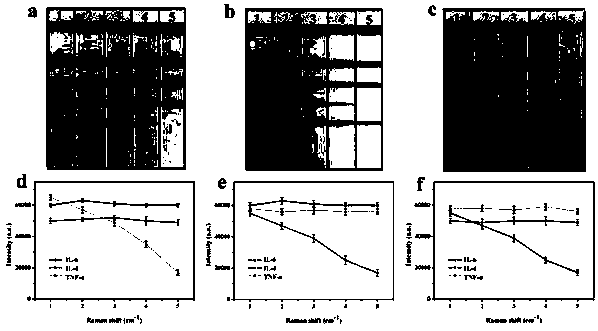
Hollow bimetallic test strip for detecting IL-6, IL-4 and TNF-alpha simultaneously, and preparation method thereof
The invention relates to a preparation method of hollow bimetallic test strip for detecting IL-6, IL-4 and TNF-alpha simultaneously. The detection problem of the IL-6, IL-4 and TNF-alpha can be effectively solved. The method comprises the following steps: preparing anti-IL-6, IL-4 and TNF-alpha protein conjugate monoclonal antibody for hollow gold-silver bimetallic mark and envelope detection lineso as to respectively prepare anti-IL-6, IL-4 and TNF-alpha protein conjugate monoclonal antibody hollow gold-silver bimetallic markers, drying and curing the hollow gold-silver bimetallic marker, and orderly enveloping the anti-IL-6, IL-4 and TNF-alpha protein conjugate monoclonal antibodies for envelope detection line on a nitrocellulose membrane to form the detection line, enveloping the anti-rat immune globulin on the nitrocellulose membrane to form a quality control line of the envelope membrane, thereby obtaining the test strip. The preparation method disclosed by the invention is simple in process, novel and unique, and capable of quickly, simply, early and effectively diagnosing and identifying stroke.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Application of serum inflammatory factors
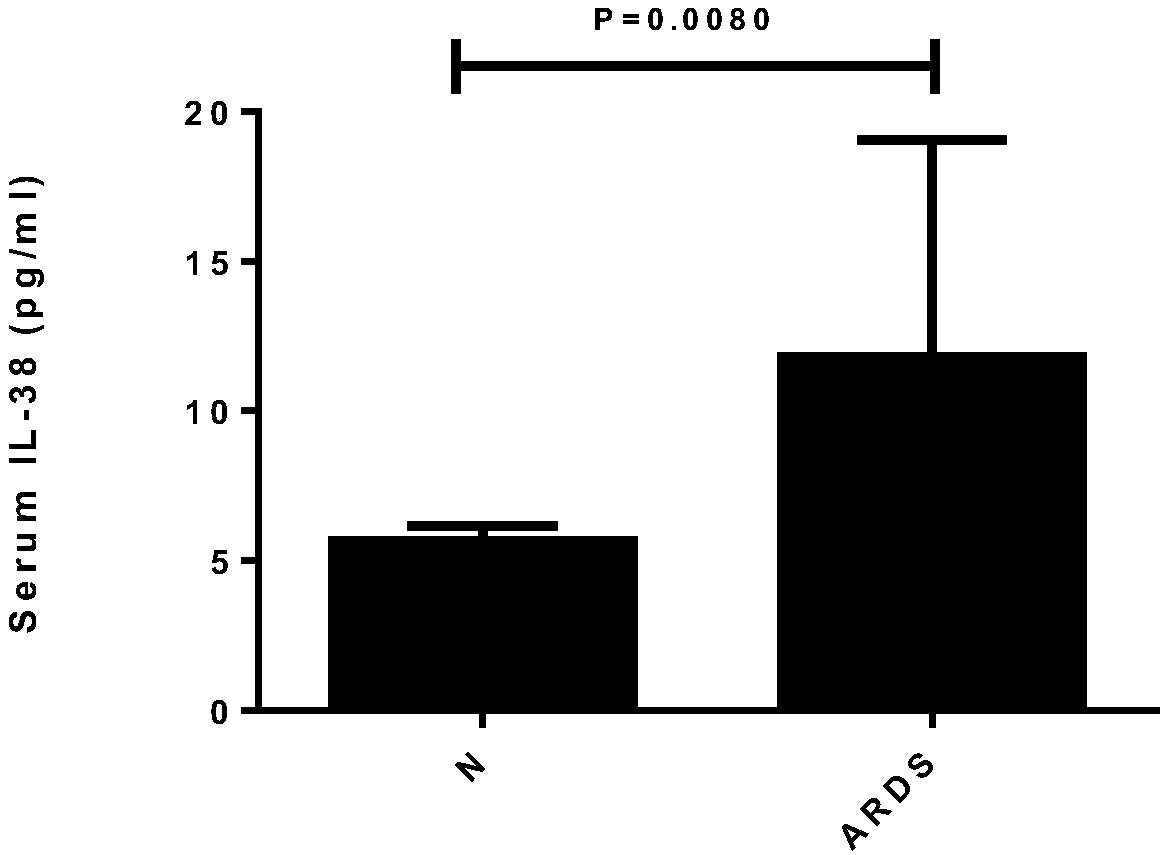
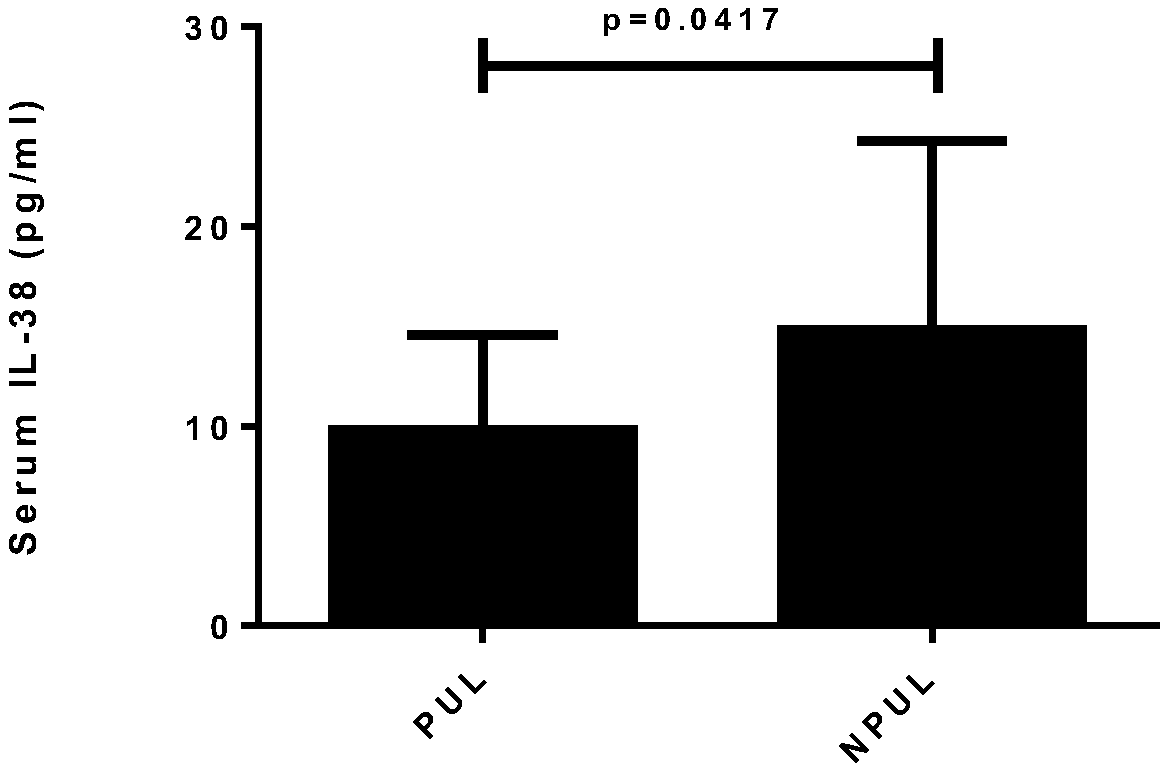
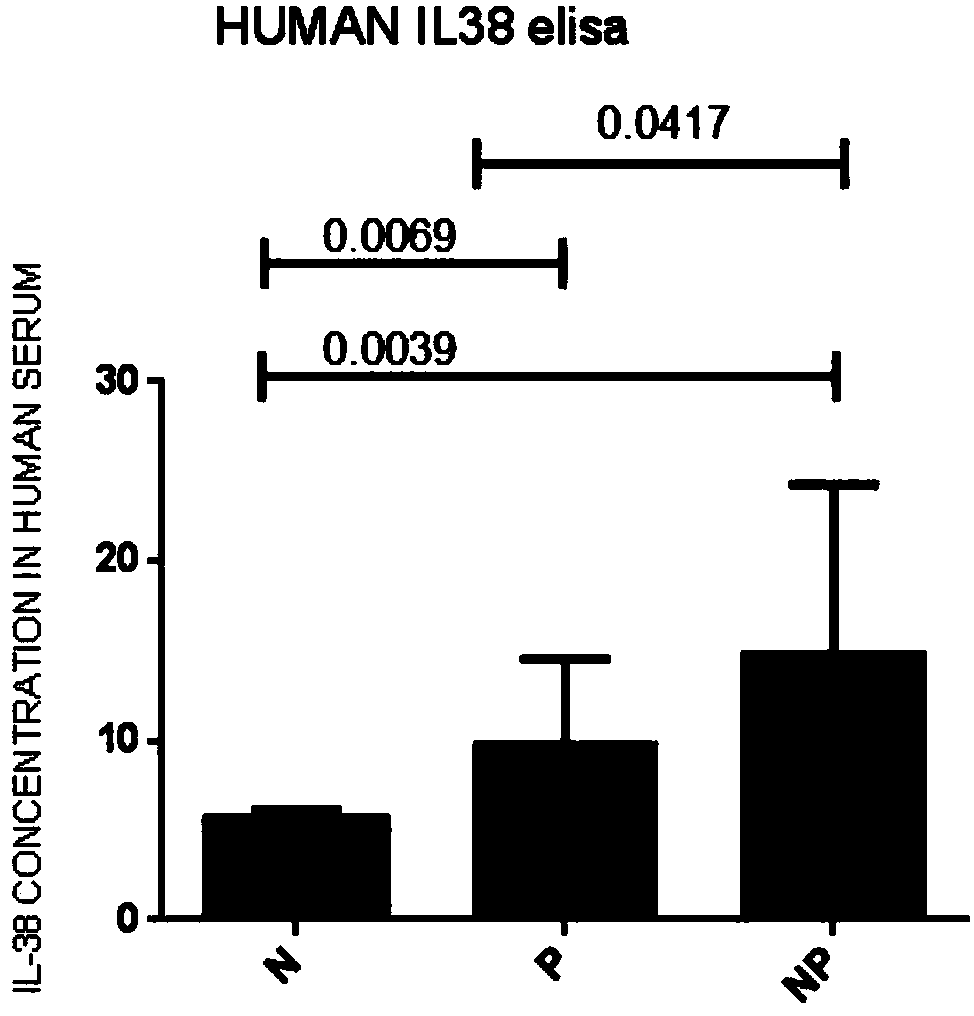
ActiveCN108333373AImprove early diagnosis rateReduce mortalityAntibacterial agentsAntimycoticsInflammatory factorsSerum ige
The invention relates to the field of medicine, particularly to application of serum inflammatory factors. Experiments show that the concentration of interleukin-38 of sepsis patients is significant in increase compared with that of healthy people. The application of serum inflammatory factors adds a reliable index for diagnosis of sepsis and provides important help for improving the promptness and accuracy of diagnosis of sepsis. The application of serum inflammatory factors aims at improve the early diagnosis rate of sepsis to achieve prompt diagnosis and treatment of sepsis and reduce the death rate of sepsis. Besides, experiments also show that the interleukin-38 achieves therapeutic effects on sepsis.
Owner:徐昉
Tumor immune biomarker and application thereof
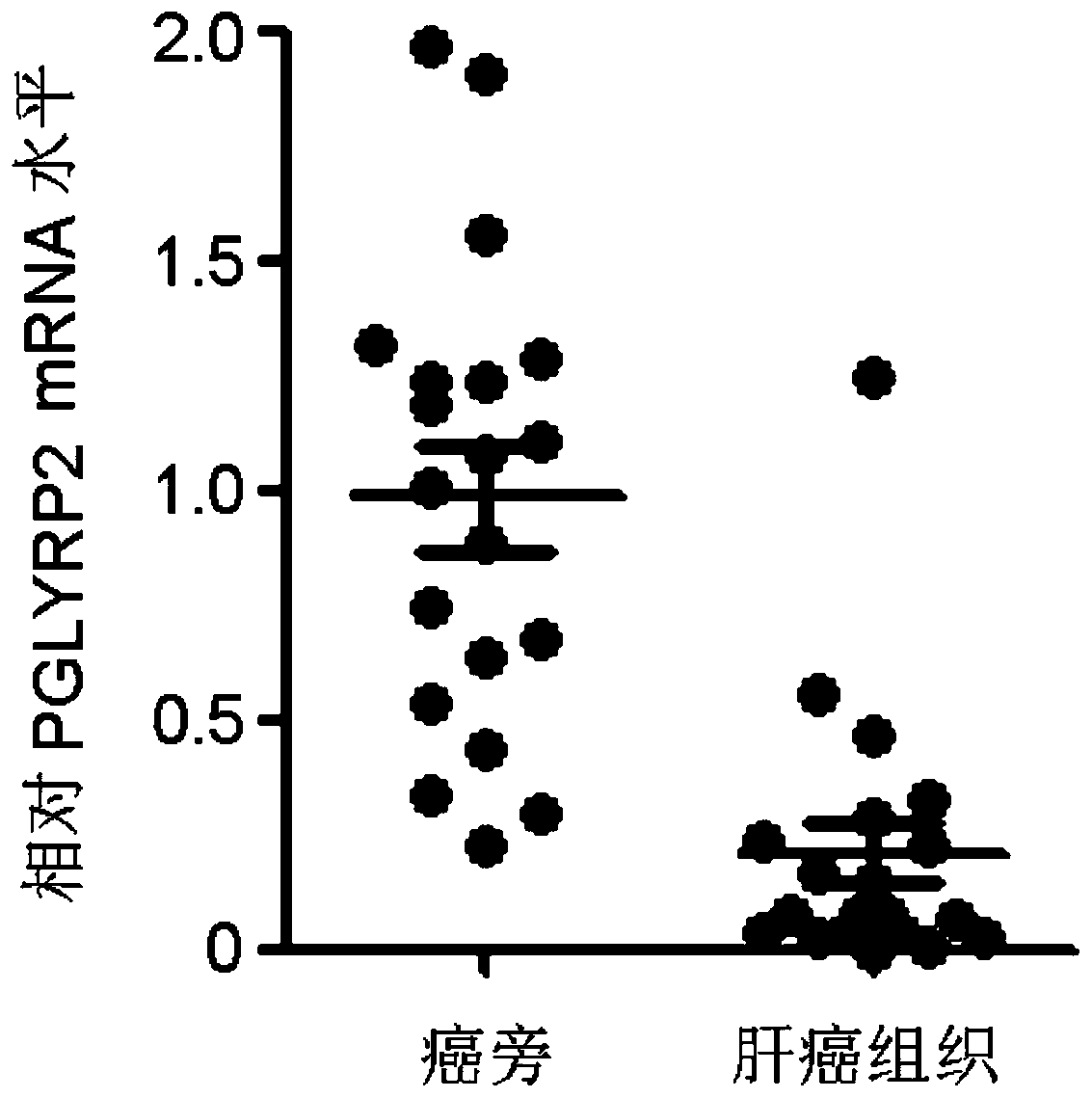
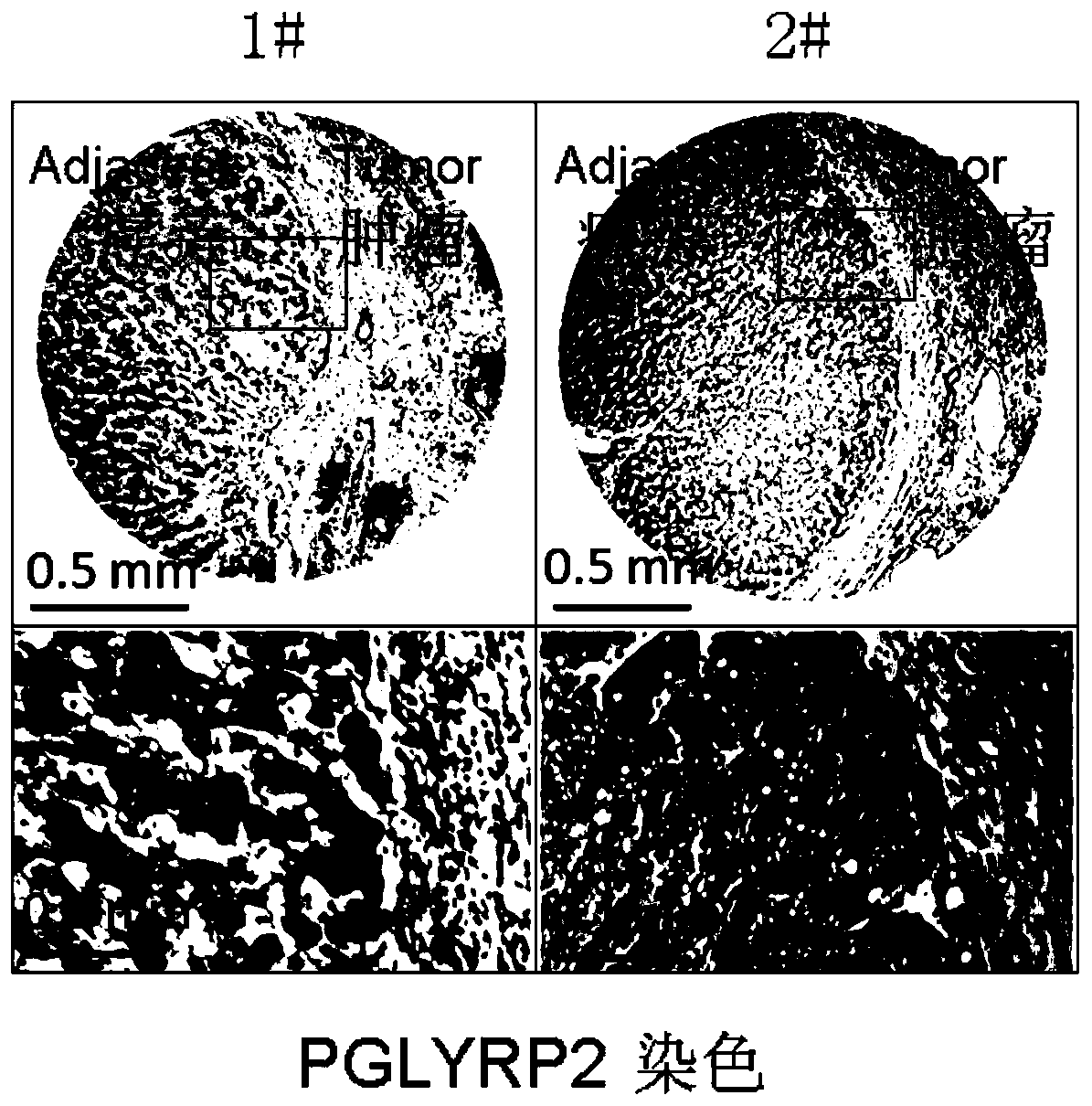
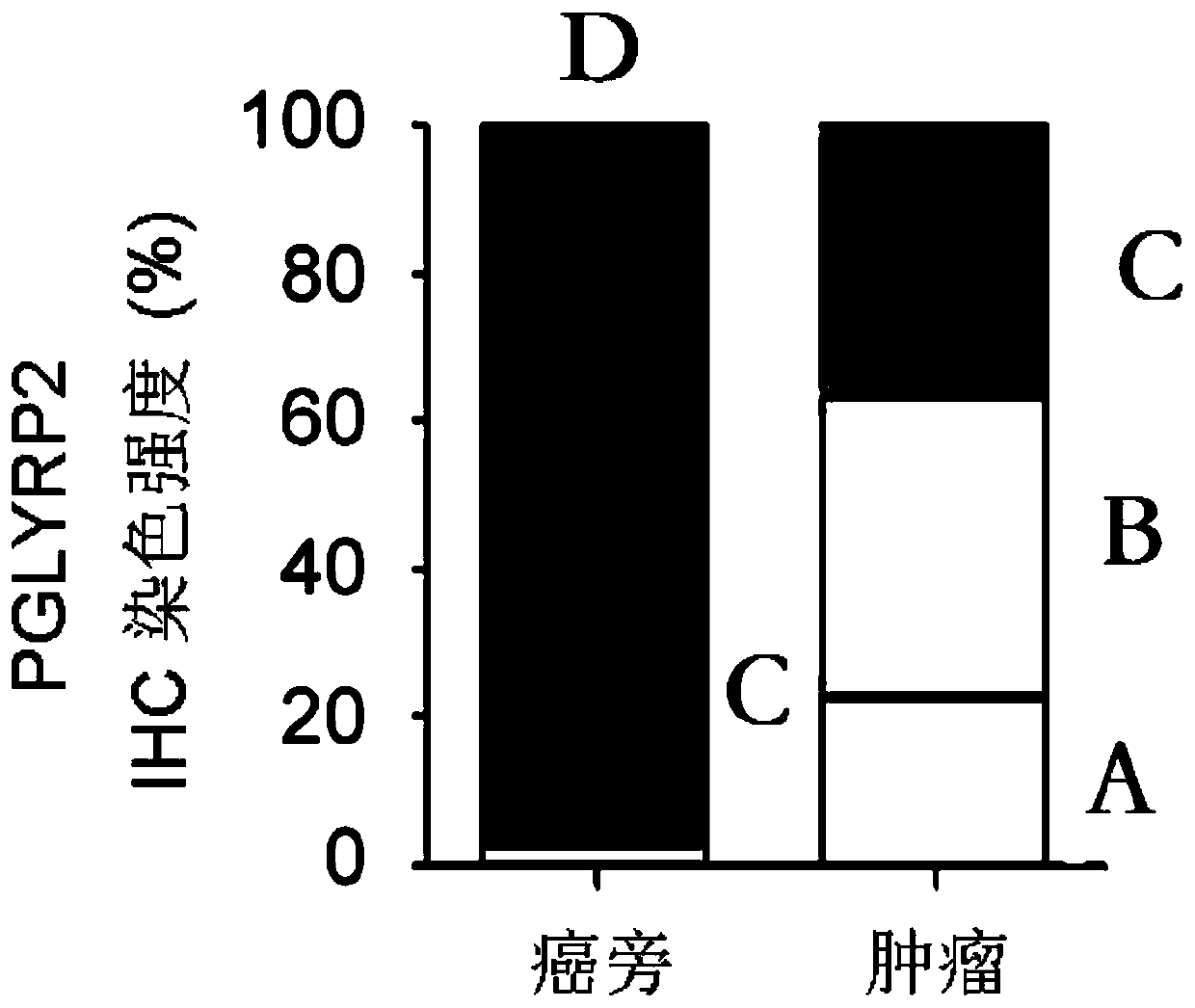
PendingCN109879956AImprove early diagnosis rateImprove response rateCell receptors/surface-antigens/surface-determinantsMicrobiological testing/measurementWilms' tumorImmunotherapy
The invention discloses a tumor immune biomarker and application thereof, relates to the technical field of biological medicine, in particular to a liver cancer tumor immune biomarker and applicationthereof. The liver cancer tumor marker comprises PGLYRP2. The invention particularly relates to application of a PGLYRP2 gene and PGLYRP2 protein in diagnosis and treatment of hepatocellular carcinoma. A new marker is provided for early diagnosis, prognosis judgment and treatment scheme selection of liver cancer by researching the relationship between expression of the PGLYRP2 gene in liver cancertissue and clinical prognosis of liver cancer patients, immune cell infiltration of tumors and induction of host anti-tumor immune response so as to improve early diagnosis rate of liver cancer patients, improve response rate of liver cancer tumor immunotherapy and improve clinical prognosis. The invention is applied to the field of oncotherapy.
Owner:HARBIN INST OF TECH
Method for establishing risk prediction model of gastric cancer
InactiveCN108504732AImprove early diagnosis rateImprove clinical outcomesMicrobiological testing/measurementNucleotideNucleotide variation
The invention relates to a method for establishing a prediction model for predicting the risk of gastric cancer, belonging to the technical field of molecular biological technology for tumors. According to the invention, the prediction model for predicting the risk of gastric cancer is established by determining a plurality of single nucleotide polymorphism (SNP) sites in collected biological samples; the established model is used for comparing and analyzing SNP sites so as to determine the gastric cancer catching risk of a population and to predict correlation of the diagnosis of gastric cancer of subjects to gastric cancer occurrence risks. The method provided by the invention is based on the established prediction model and biological samples collected from subjects, analyzes statistically-significant single nucleotide variation by contrasting a normal population and patients with gastric cancer and taking the frequency of genovariation into consideration, and determines the correlation of the diagnosis of gastric cancer of the subjects to gastric cancer occurrence risks, so an early diagnosis rate and clinical outcome are improved. The risk prediction model is applicable to prediction of the gastric cancer catching risks of people.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
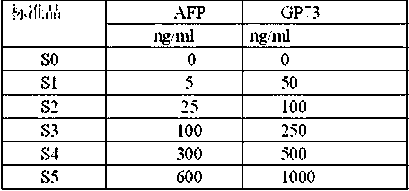
Liver cancer tumour marker AFP, AFP variant and GP73 antigen synchronous immunodetection kit, method and application
The invention relates to a liver cancer tumour marker AFP, AFP variant and GP73 antigen synchronous immunodetection kit, a method and application, and belongs to the technical field of immunodetection. The kit comprises magnetic particles, AFP and GP73 antibody coupled mixed acridinium ester, GP73, an AFP calibration product, first stimulation solution, second stimulation solution, washing solution and all kinds of diluting solution; and the magnetic particles are respectively marked by AFP, LCA-BSA and GP73. By adoption of the kit and the detection method, the sensitivity, the specificity andthe accuracy are high; the detection time is short; identification on benign liver diseases and malignant liver diseases and early diagnosis on liver cancers can be easily carried out; and the kit and the detection method have an important purpose for increasing the early diagnosis rate of patients with liver cancers and prolonging the survival rate, and have wide clinical application value.
Owner:万东山
Application of magnetic resonance targeted prostate puncture in prostate cancer diagnosis
PendingCN112690776AImprove early diagnosis rateImprove diagnosis rateSurgical needlesVaccination/ovulation diagnosticsSagittal planeNeedle puncture
The invention discloses application of magnetic resonance targeted prostate puncture in prostate cancer diagnosis. The which comprises the following steps of: marking puncture targets on MRI and T2W TSE sequence images of a plurality of high-resolution sections of a prostate, displaying a sagittal plane reconstruction image of the MRI and a sagittal plane image of transrectal ultrasound on the same screen to ensure that the images are fused and displayed synchronously, and adding 3 needles to the targets on the basis of 13-needle puncture. The invention relates to the technical field of biological diagnosis application, and particularly provides the application of magnetic resonance targeted prostate puncture in prostate cancer diagnosis, which can accurately guide a puncture gun to perform targeted puncture on a target point by a magnetic resonance and transrectal prostate color Doppler ultrasound image fusion technology so as to improve the early diagnosis rate of prostate cancer.
Owner:HENAN UNIVERSITY
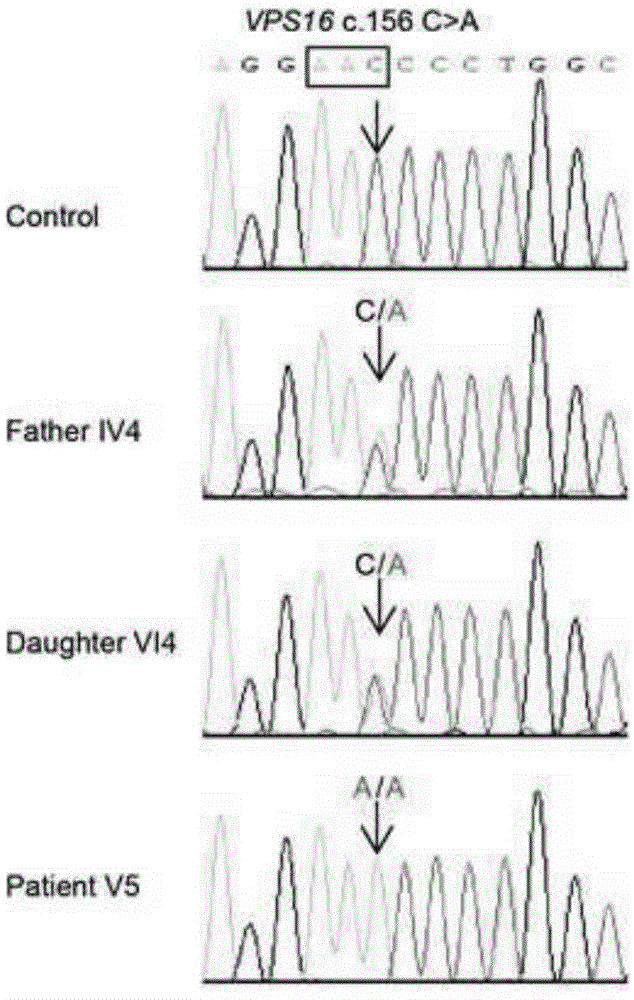
Detection primers, method and kit for myodystonia VPS16 gene
InactiveCN104388553AAvoid one-sidednessImprove early diagnosis rateMicrobiological testing/measurementDNA/RNA fragmentationForward primerMutation detection
The invention provides detection primers for a myodystonia VPS16 gene. The primers are characterized by comprising a forward primer P1 with a base sequence shown in SEQ.ID.No.1 in a sequence table and a reverse primer P2 with a base sequence shown in SEQ.ID.No.2 in the of sequence table. By adopting the detection primers, DNA of a standard sample can be directly amplified to obtain VPS16 gene to be analyzed and judged, the one-sidedness of conventional mutation detection such as TOR1A, THAP1 and GCH1 is overcome, the process can be completed through an ordinary PCR method directly, and the detection primers have the advantages of rapidness, accuracy, high efficiency, simplicity, convenience, high early-stage diagnostic rate and the like; and the detection results can provide scientific assistance for early-stage diagnosis and differential diagnosis for myodystonia and development of myodystonia treatment medicine.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN +1
Molecular markers for auxiliary diagnosis of viral meningitis as well as application and kit of molecular markers
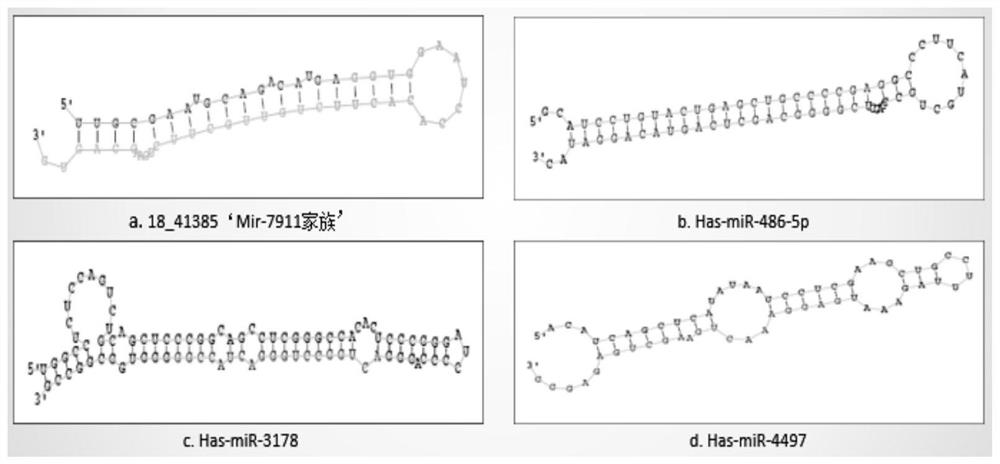
ActiveCN112779329AVarious clinical manifestationsImprove early diagnosis rateMicrobiological testing/measurementDNA/RNA fragmentationTreatment effectGene Microarray
The invention discloses molecular markers for auxiliary clinical diagnosis of viral meningitis as well as application and a kit of the molecular markers. Firstly, systematic gene chip analysis is carried out on two groups of cerebrospinal fluid samples of viral meningitis and normal control by a miSeq high-throughput sequencing technology, differential miRNA expression is screened, the screened differential expression miRNA is further verified one by one by an RT-qPCR technology, and finally, it is found that miR-RNA 18_41385, hsa-miR-486-5p, hsa-miR-4497 and the hsa-miR-3178 can be used as specific markers for early auxiliary clinical diagnosis of the viral meningitis. The markers can also be used for differential diagnosis of other meningitis. The kit has the advantages of being good in stability, simple and convenient in detection method, high in sensitivity, specific and the like, achieves effective supplementation of an existing clinical viral meningitis diagnosis method and rapid definite diagnosis of viral meningitis patients, and is of great significance in reducing the death rate of the viral meningitis and improving the treatment effect and prognosis.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
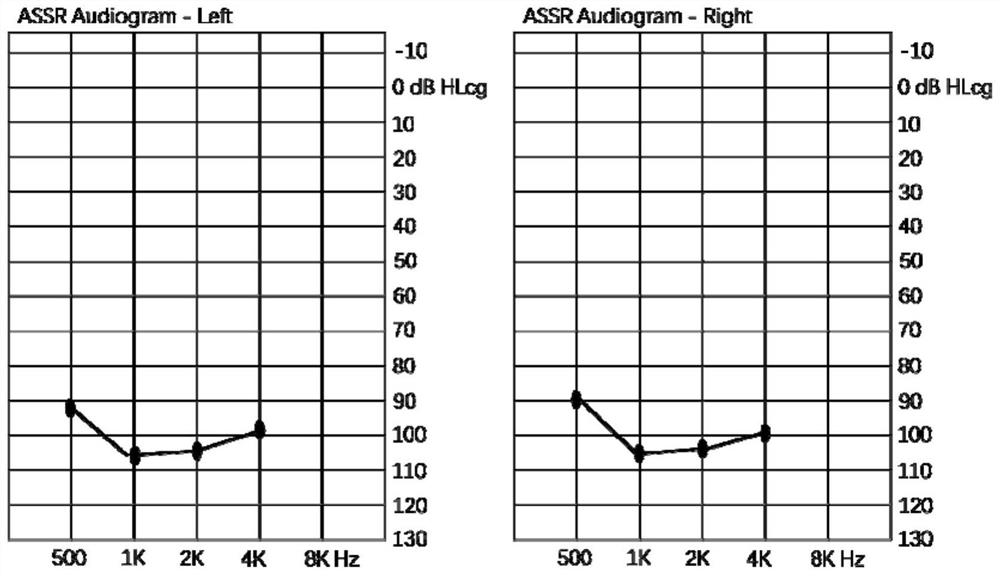
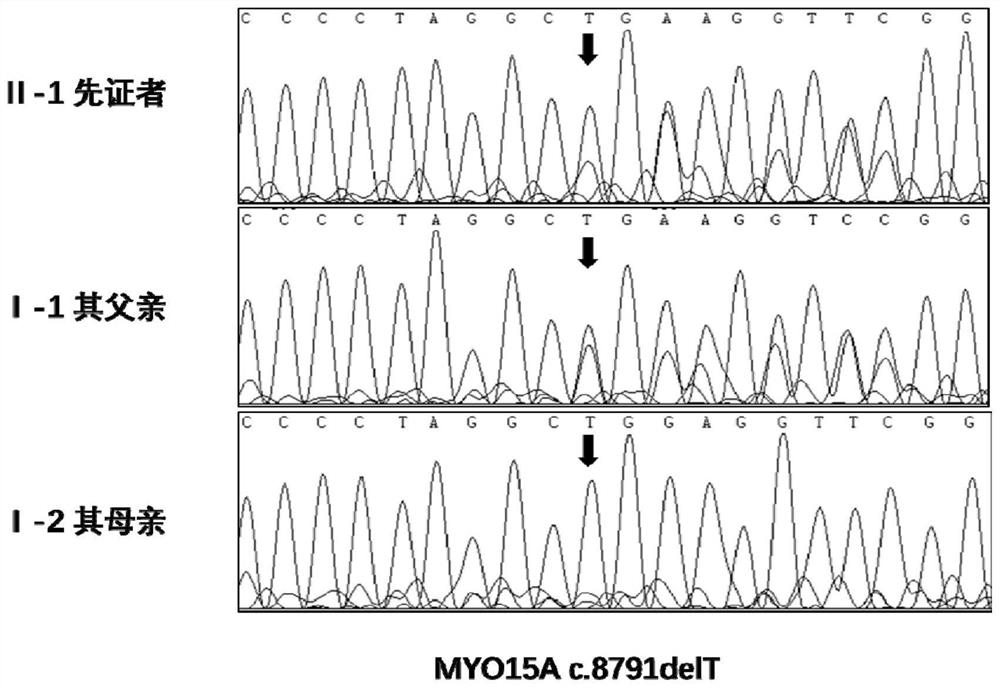
MYO15A gene mutant and application thereof
PendingCN112522275AImprove early diagnosis rateSenses disorderGenetic material ingredientsHL - Hearing lossDisease
The invention provides a non-syndromic hearing loss related gene mutant and application thereof, and particularly provides an MYO15A gene mutant and application thereof. Compared with a wild type MYO15A gene, the gene mutant has the c.10419-10423 del CAGCT mutation and / or the c.8791 del T mutation. The gene mutant is detectable, and by detecting whether the gene mutant is present in a biological sample or not, whether a biological sample suffers from non-syndromic hearing loss or not can be effectively detected. Detection and research of the hereditary hearing loss disease are expanded and perfected through discovery of the gene mutant, and a new detection site and a new detection method and way are provided for diagnosis or treatment of the disease.
Owner:BGI GENOMICS CO LTD +1
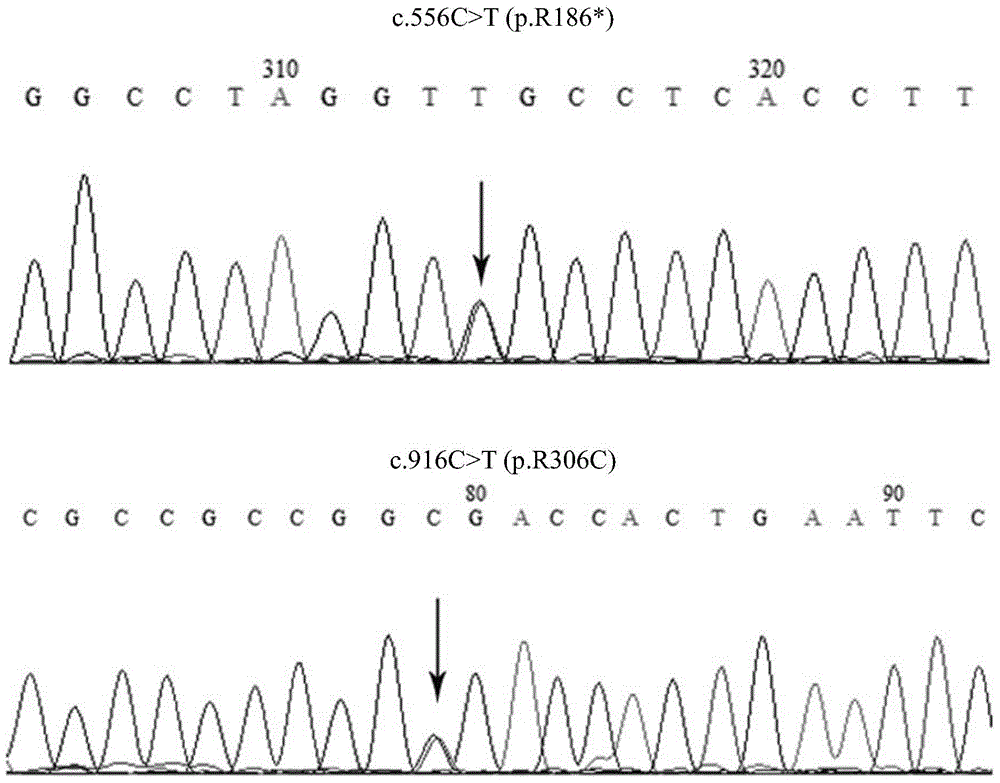
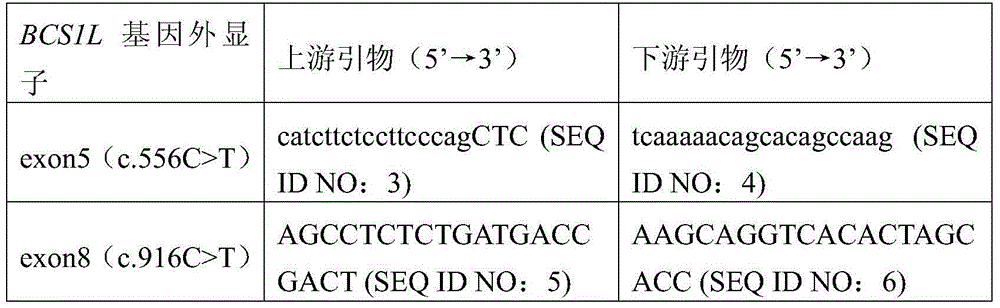
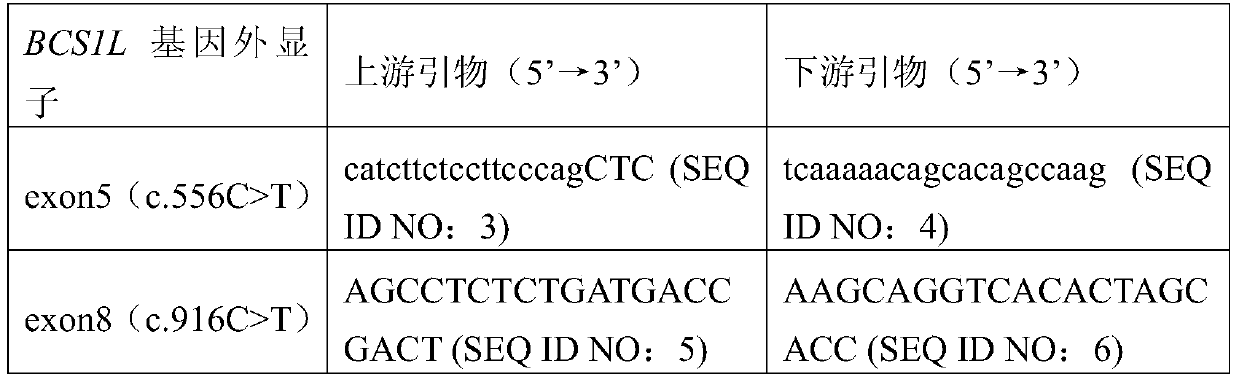
BCS1L gene mutant and use thereof
ActiveCN105802974AImprove early diagnosis rateBioreactor/fermenter combinationsBiological substance pretreatmentsMutantBjörnstad syndrome
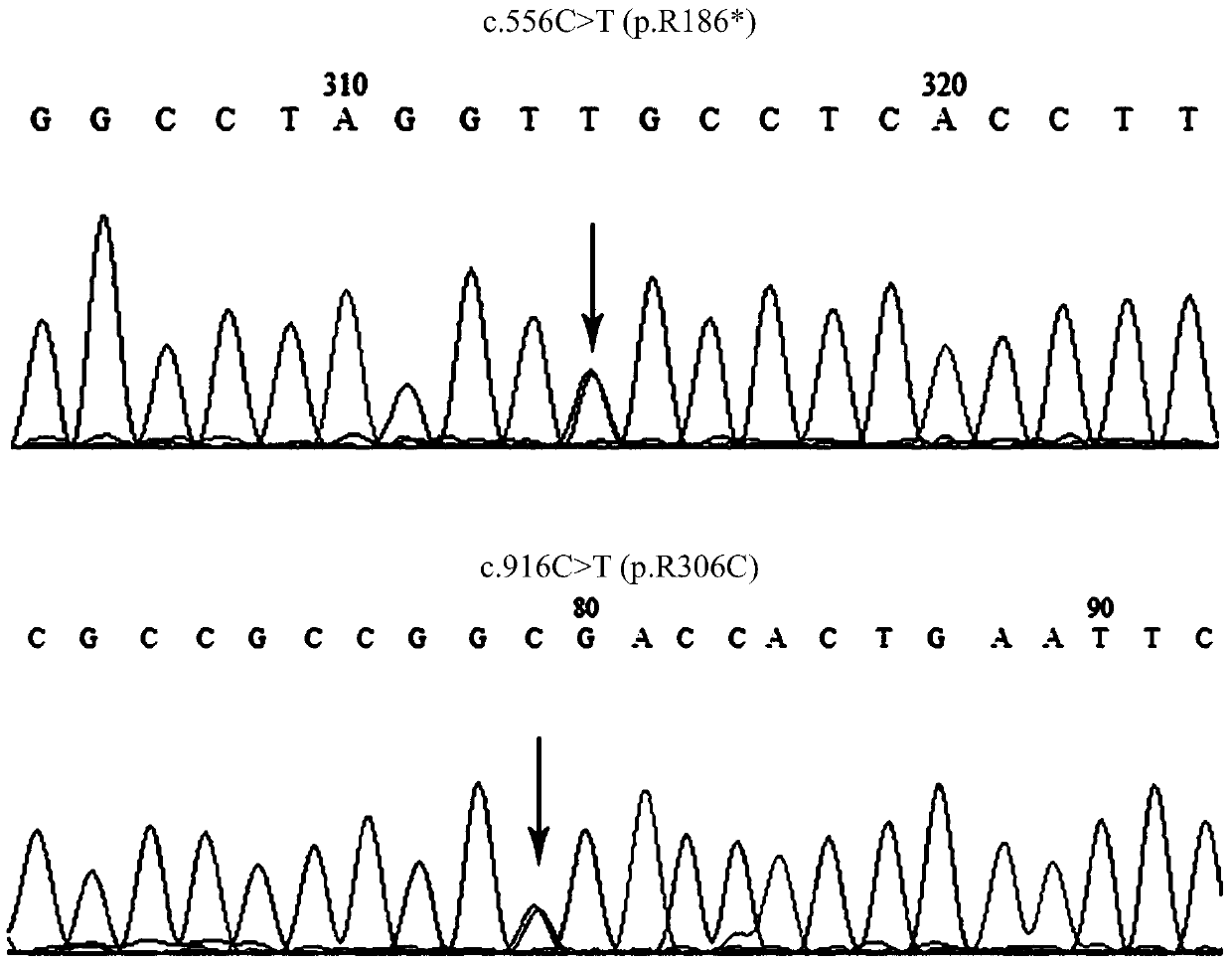
The invention discloses a BCS1L gene mutant and a use thereof and concretely relates to a separated nucleic acid for coding a BCS1L mutant, a separated polypeptide, a method for screening a biological sample easily suffering from Bjornstad syndrome, a system for screening a biological sample easily suffering from Bjornstad syndrome and a kit for screening a biological sample easily suffering from Bjornstad syndrome. Compared with a nucleic acid shown in the formula of SEQ ID NO: 1, the separated nucleic acid for coding a BCS1L mutant has c.556C>T mutation or c. 916C>T mutation. Through detection of existence of the novel mutant in a biological sample, the BCS1L gene mutant can effectively detect that if the biological sample easily suffers Bjornstad syndrome.
Owner:BGI SHENZHEN CO LTD
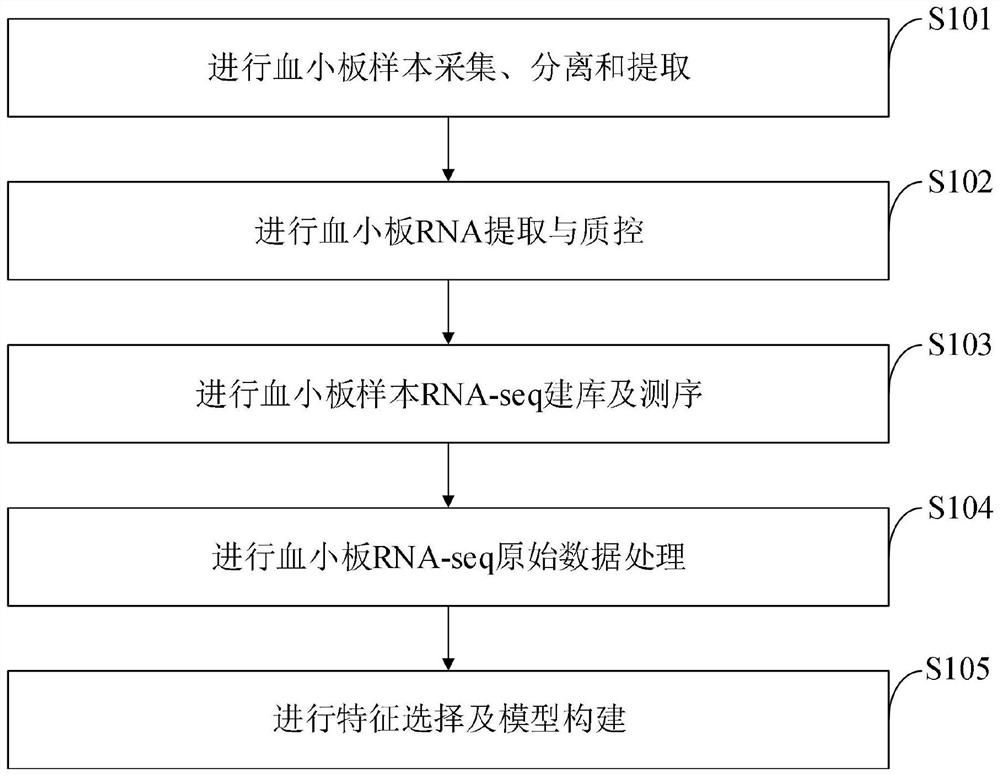
Diagnosis model of platelet 102 gene for ovarian cancer, construction method and application
InactiveCN114634969AImprove long-term poor prognosisThe extraction method is convenient and efficientMedical simulationMicrobiological testing/measurementRNA extractionBiologic marker
The invention belongs to the technical field of biomarkers, and discloses a platelet 102 gene diagnosis model for ovarian cancer as well as a construction method and application thereof, and the construction method of the platelet 102 gene diagnosis model for ovarian cancer comprises the following steps: collecting, separating and extracting a platelet sample; carrying out platelet RNA extraction and quality control; carrying out RNA-seq library establishment and sequencing on the platelet samples; platelet RNA-seq original data processing is carried out; and carrying out feature selection and model construction. Compared with CA125, the diagnosis performance of the diagnosis model TEPOC based on the platelet 102 gene constructed by the invention is improved to different degrees in an internal verification set (DC) and three external verification sets (VC1, VC2 and VC3), and the combination of TEPOC and CA125 can further improve the diagnosis performance of ovarian cancer. In addition, the present invention (TEPOC) is also superior to CA125 in distinguishing endometriosis from ovarian cancer and identifying the manifestations of patients with border, early and non-epithelial ovarian cancer.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
I<131> labeled anti-human neuropilin receptor-2 monoclonal antibody E4 and application thereof
InactiveCN107412795AImprove targetingImprove Molecular Imaging EfficiencyPharmaceutical delivery mechanismRadioactive preparation carriersTherapeutic effectIodination reaction
The invention relates to an I<131> labeled anti-human neuropilin receptor-2 (NRP-2) monoclonal antibody E4. The antibody E4 is labeled by I<131> through a chloramine T labeling method. The I<131>-E4 is produced through the following steps: 1) preparing the antibody E4, NaI<131>, and PBS being 7.4-7.6 in pH value, and adding the components into an EP tube, adding newly-prepared chloramine T and rapidly vibrating and uniformly mixing the liquid, performing a reaction at room temperature, and adding a newly-prepared reduction agent, sodium metabisulfite, to terminate the iodination reaction; 2) moving out the reaction liquid, and measuring the labeling rate of a labeled product through TLC, normal saline being a developing solvent; 3) treating the reaction liquid on a Sephadex G25 column to purify the reaction liquid with PBS being about 7.4 in pH value as an eluting solution to prepare I<131>-E4. Advantages of the radionuclide are integrated with the antitumor cell humanized monoclonal antibody, so that the targeting property and molecular imaging effect of the medicine are improved. The antibody E4 can increase the early-stage diagnosis rate and monitor treatment effect of positive tumor expressed by the NRP-2, has a wider available range and is suitable for imaging positive tumors expressed by the NRP-2 in all stages.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
AMSH gene mutant and application thereof
ActiveCN105821062AImprove early diagnosis rateBioreactor/fermenter combinationsBiological substance pretreatmentsGene MutantMarie disease
The invention discloses an AMSH gene mutant and an application thereof, and concretely relates to isolated nucleic acid for coding the AMSH mutant, isolated nucleic acid, a method for screening a biological sample predisposed to charcot-marie-tooth disease, a system for screening the biological sample predisposed to charcot-marie-tooth disease, and a kit used for screening the biological sample predisposed to charcot-marie-tooth disease. Compared with SEQ ID NO:1, the isolated nucleic acid for coding the AMSH mutant comprises c.364A>G mutation. By detecting whether the new mutant exists in the biological sample or not, whether the biological sample is predisposed to charcot-marie-tooth disease or not can be effectively detected.
Owner:BGI SHENZHEN CO LTD +1
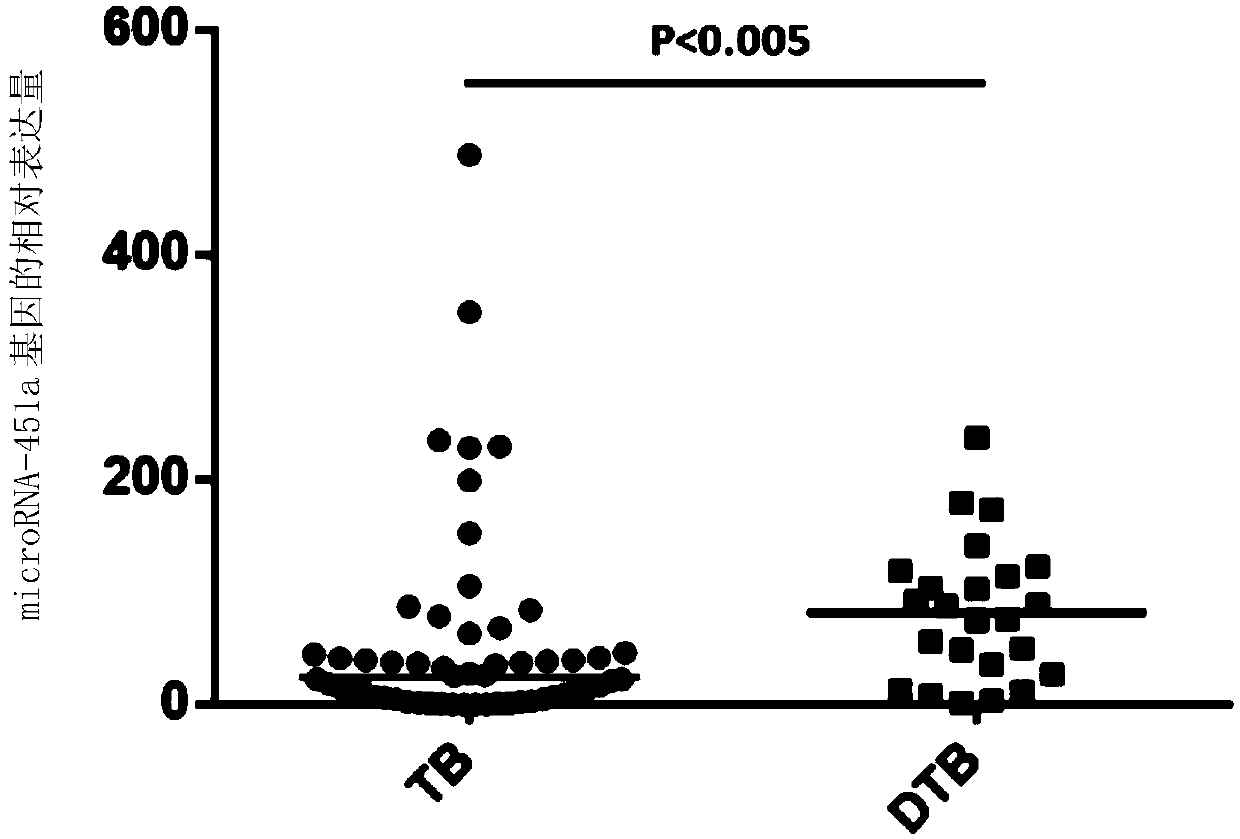
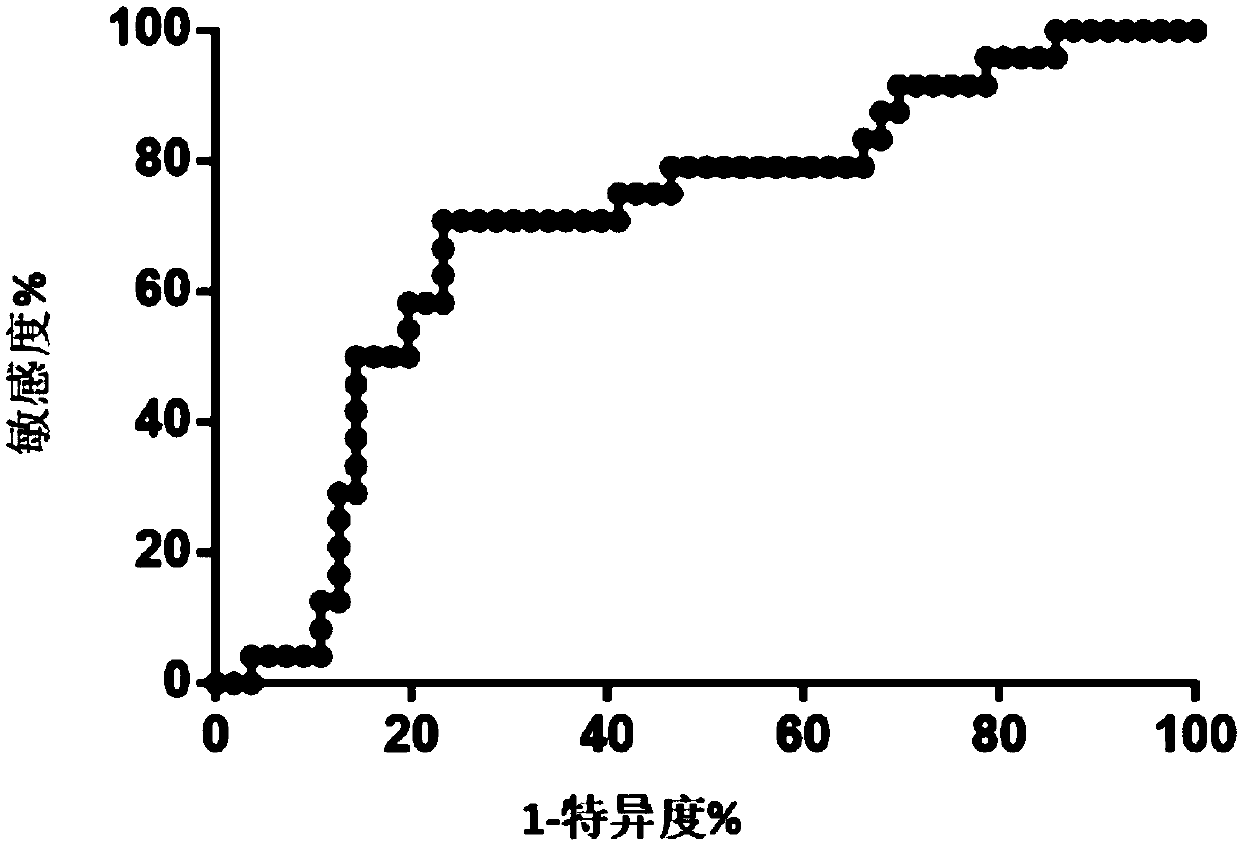
Kit for assisting in diagnosing hematogenous disseminated pulmonary tuberculosis
ActiveCN109609620AImprove early diagnosis rateHigh diagnostic specificityMicrobiological testing/measurementMicroRNAPulmonary tuberculosis
The invention discloses a kit for assisting in diagnosing hematogenous disseminated pulmonary tuberculosis. The present invention also includes applications of a substance for detecting microRNA-451aabundance, a substance for detecting microRNA-451a relative level, a substance for detecting microRNA-451a gene, a substance for detecting microRNA-451a gene expression level, and a substance for detecting microRNA-451a gene relative expression level in preparing a kit. The purpose of the kit is to diagnose or assist in diagnosing hematogenous disseminated pulmonary tuberculosis; to diagnose or assist in diagnosing hematogenous disseminated tuberculosis from pulmonary tuberculosis; and to identify or assist in identifying secondary pulmonary tuberculosis and hematogenous disseminated pulmonarytuberculosis. The inventor of the present invention find in research work that microRNA-451a is associated with the occurrence of hematogenous disseminated pulmonary tuberculosis, and can predict theoccurrence of hematogenous disseminated pulmonary tuberculosis and provide a reliable basis for timely adjustment of clinical treatment plans.
Owner:BEIJING TUBERCULOSIS & THORACIC TUMOR RES INST
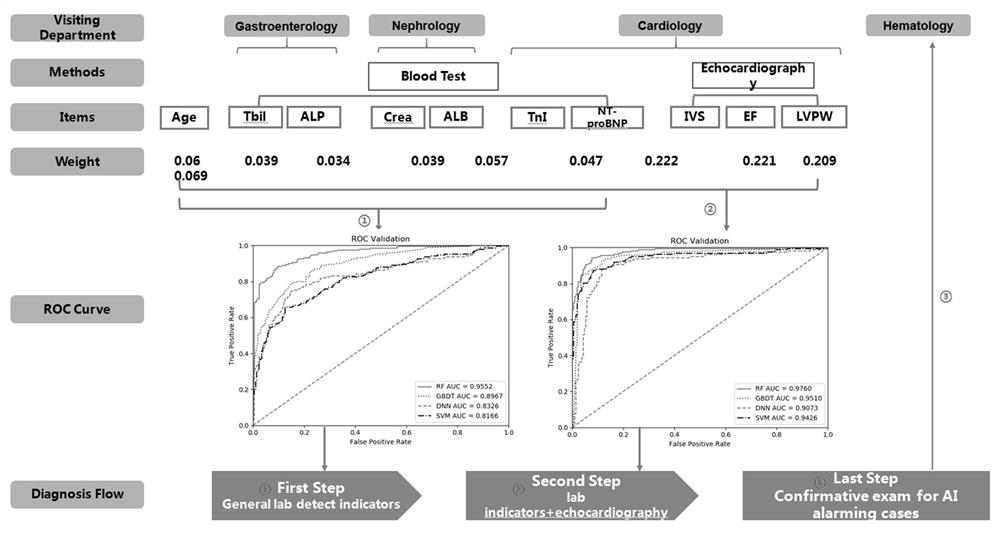
Method for establishing early screening light-chain amyloidosis based on machine learning and application thereof
PendingCN113744869AImprove accuracyAdequate hematologic remissionMedical data miningMedical automated diagnosisArtificial intelligenceEarly warning model
The invention belongs to the technical field of early screening and artificial intelligence of light-chain amyloidosis, and particularly relates to a method for establishing an artificial intelligence auxiliary system for early screening of light-chain amyloidosis based on machine learning in combination with clinical routine assay and echocardiography and application of the artificial intelligence auxiliary system. According to the conventional test results of 1064 clinical cases of light-chain amyloidosis and non-AL amyloidosis (heart failure, cardiomyopathy, liver diseases and kidney diseases), an early auxiliary screening model of the AL amyloidosis is established by using RF, SVM, DNN and GBDT, and the prediction possibility accuracy can reach 90% or above. The early warning model has the characteristics of easiness in popularization and convenience in use, can greatly improve the cognition of light-chain amyloidosis in primary hospitals and the early screening of patients, and has wide application prospects and profound clinical significance.
Owner:SHENGJING HOSPITAL OF CHINA MEDICAL UNIV
bcs1l gene mutant and its application
ActiveCN105802974BImprove early diagnosis rateBioreactor/fermenter combinationsBiological substance pretreatmentsBCS1LNew mutation
The invention discloses BCS1L gene mutants and applications thereof, in particular to isolated nucleic acids encoding BCS1L mutants, isolated polypeptides, methods for screening biological samples susceptible to syndromes, systems for screening biological samples susceptible to syndromes, and Kit for screening biological samples for predisposition to syndromes. Wherein, compared with SEQ ID NO: 1, the isolated nucleic acid encoding the BCS1L mutant has c.556C>T mutation, or c.916C>T mutation. By detecting whether the new mutant exists in the biological sample, it is possible to effectively detect whether the biological sample is susceptible to the syndrome.
Owner:BGI SHENZHEN CO LTD
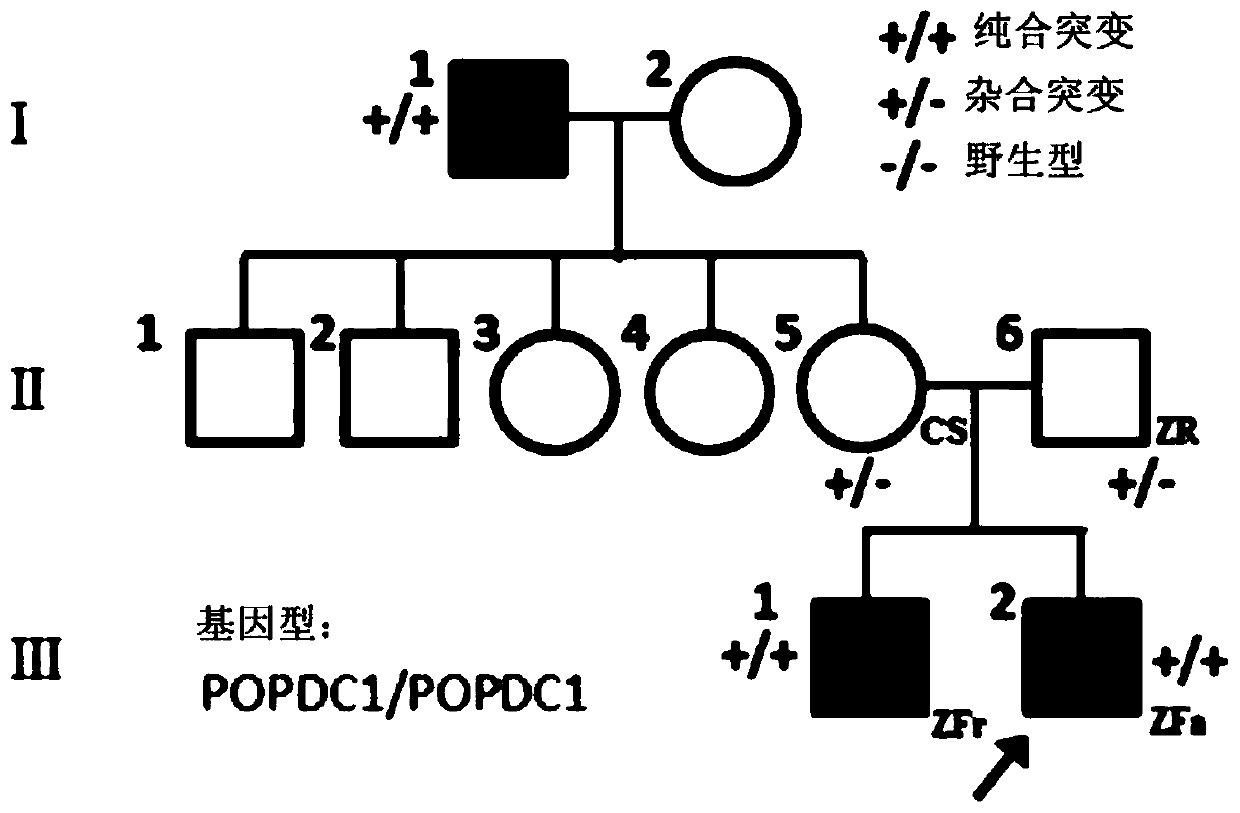
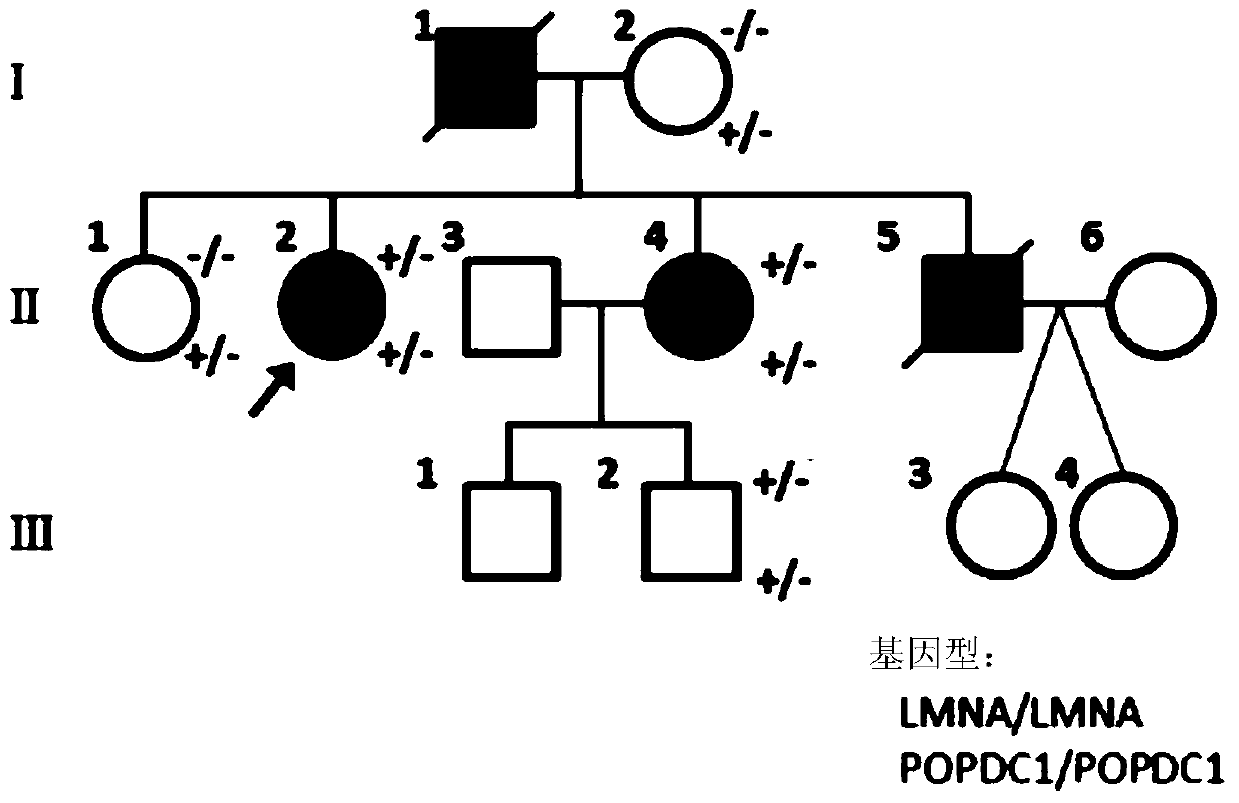
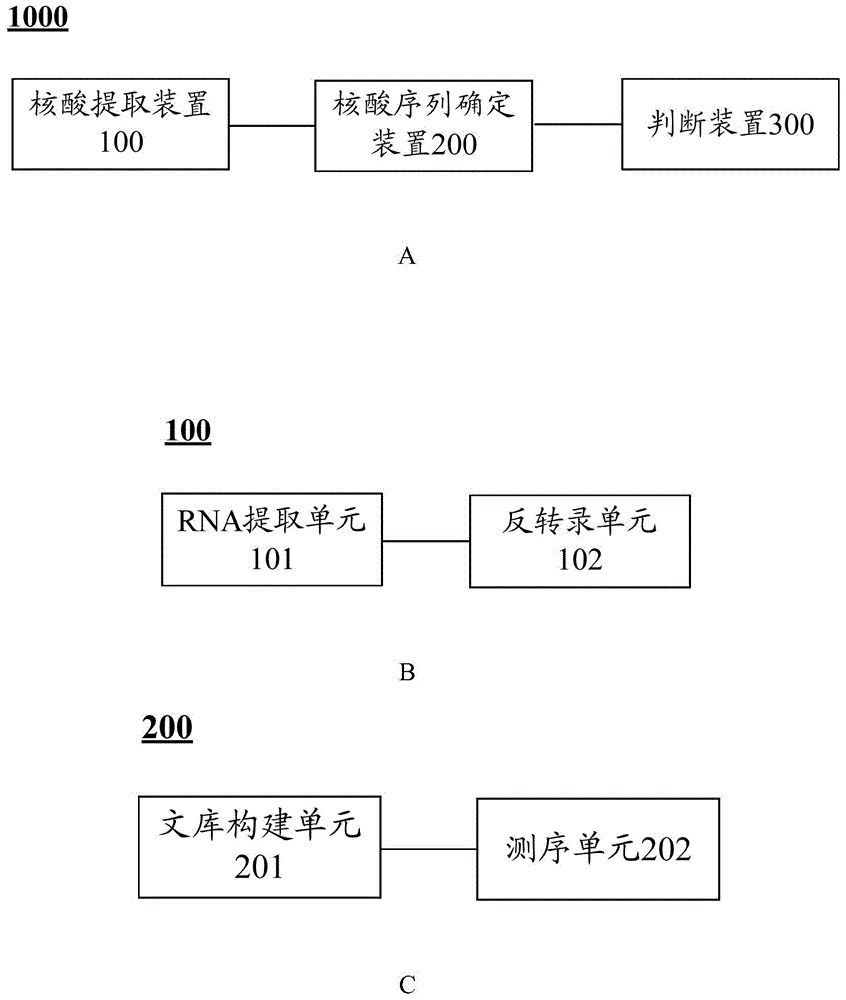
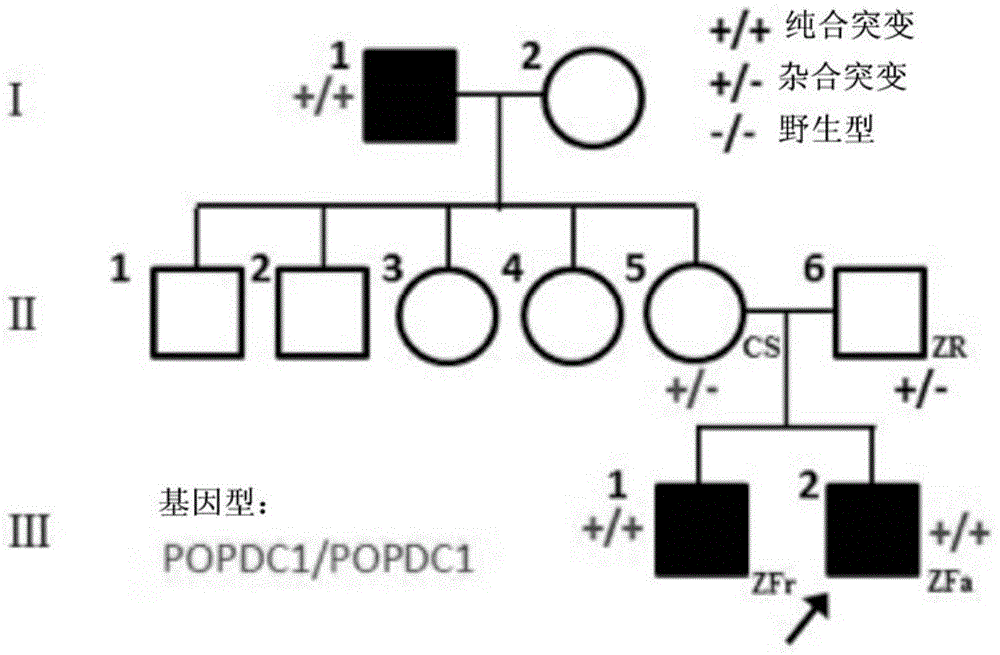
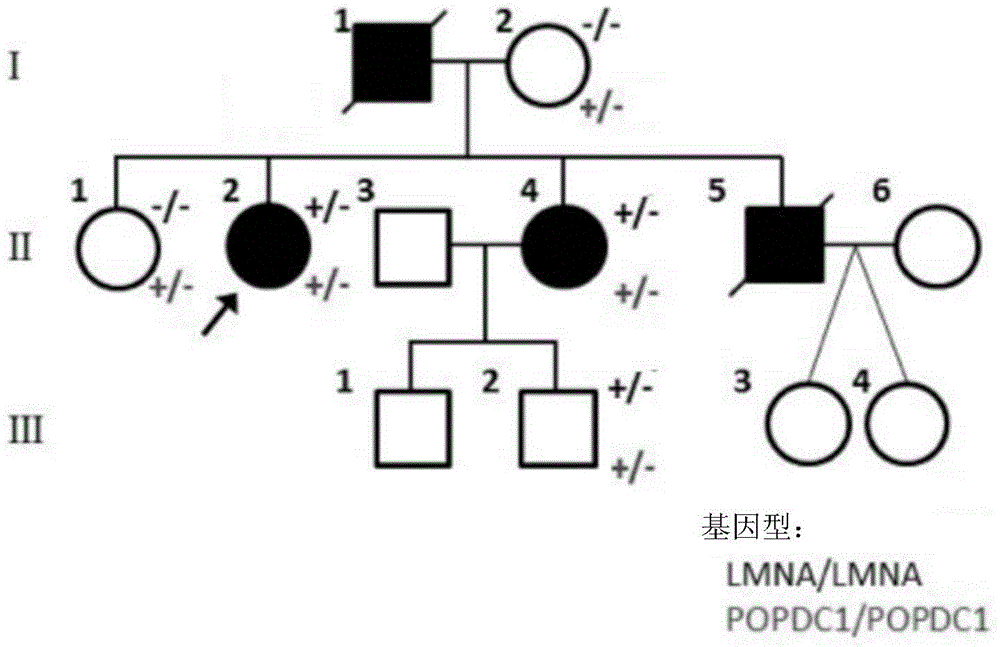
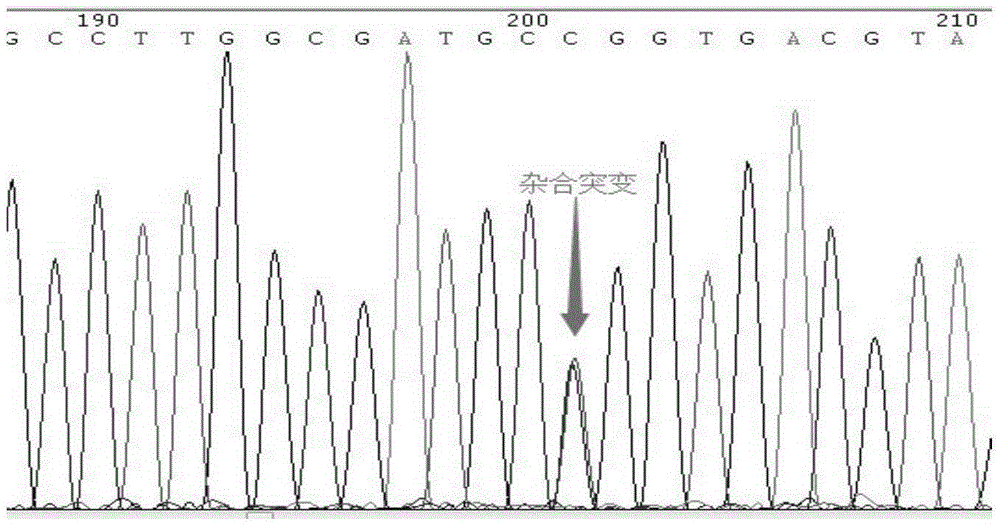
Isolated nucleic acids encoding popdc1 mutants and uses thereof
ActiveCN105821047BImprove early diagnosis rateBioreactor/fermenter combinationsBiological substance pretreatmentsMuscular dystrophyMedicine
The invention discloses a POPDC1 gene mutant and an application thereof, and more specifically relates to isolated nucleic acid coding the POPDC1 mutant, isolated polypeptide, a system for screening a biological sample predisposed to limb-girdle muscular dystrophy, a kit used for screening the biological sample predisposed to limb-girdle muscular dystrophy, and a method for constructing a medicine screening model. Compared with SEQ ID NO:1, the isolated nucleic acid coding the POPDC1 mutant comprises at least one of c.602C>T mutation, c.515G>A mutation, c.385C>T mutation, and c.427A>G mutation. By detecting whether the new mutant exists in the biological sample or not, whether the biological sample is predisposed to limb-girdle muscular dystrophy symptom or not can be effectively detected.
Owner:石家庄华大医学检验实验室有限公司 +1
Isolated nucleic acid coding POPDC1 mutant and application thereof
ActiveCN105821047AImprove early diagnosis rateBioreactor/fermenter combinationsBiological substance pretreatmentsMuscular dystrophyGene Mutant
The invention discloses a POPDC1 gene mutant and an application thereof, and more specifically relates to isolated nucleic acid coding the POPDC1 mutant, isolated polypeptide, a system for screening a biological sample predisposed to limb-girdle muscular dystrophy, a kit used for screening the biological sample predisposed to limb-girdle muscular dystrophy, and a method for constructing a medicine screening model. Compared with SEQ ID NO:1, the isolated nucleic acid coding the POPDC1 mutant comprises at least one of c.602C>T mutation, c.515G>A mutation, c.385C>T mutation, and c.427A>G mutation. By detecting whether the new mutant exists in the biological sample or not, whether the biological sample is predisposed to limb-girdle muscular dystrophy symptom or not can be effectively detected.
Owner:石家庄华大医学检验实验室有限公司 +1
Early-onset diabetes gene mutant and applications thereof
ActiveCN105255902AImprove early diagnosis rateBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseNucleotide
The invention discloses an early-onset diabetes gene mutant, of which the nucleotide sequence is shown in SEQ ID NO.1. The invention also discloses an early-onset diabetes gene mutant encoded protein, of which the amino acid sequence is shown in SEQ ID NO.2. The invention also discloses a system for screening biological samples susceptible to early-onset diabetes, and according to the invention, a new virulence gene of early-onset diabetes (mody) is found. On the basis, the system can be used for early screening virulence mutant gene carriers of early-onset diabetes (mody), and then the carriers can be subjected to early intervention treatment before catching the early-onset diabetes; and the system also can be applied to the molecular diagnosis of patients with early-onset diabetes and the differential diagnosis of related diseases. The technique has the advantages of rapidness, accuracy, high efficiency, simplicity, convenience, high early diagnostic rate and the like, and detection results can provide a scientific basis for the early diagnosis and differential diagnosis of early-onset diabetes (mody) and the development of medicaments for the treatment of early-onset diabetes (mody).
Owner:XINJIANG MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com