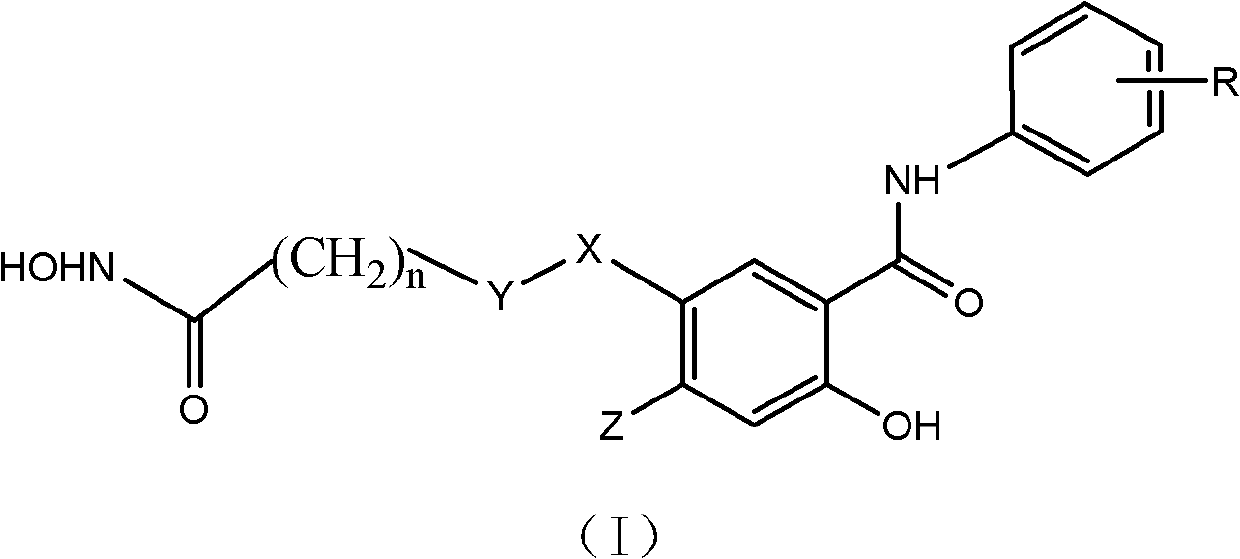
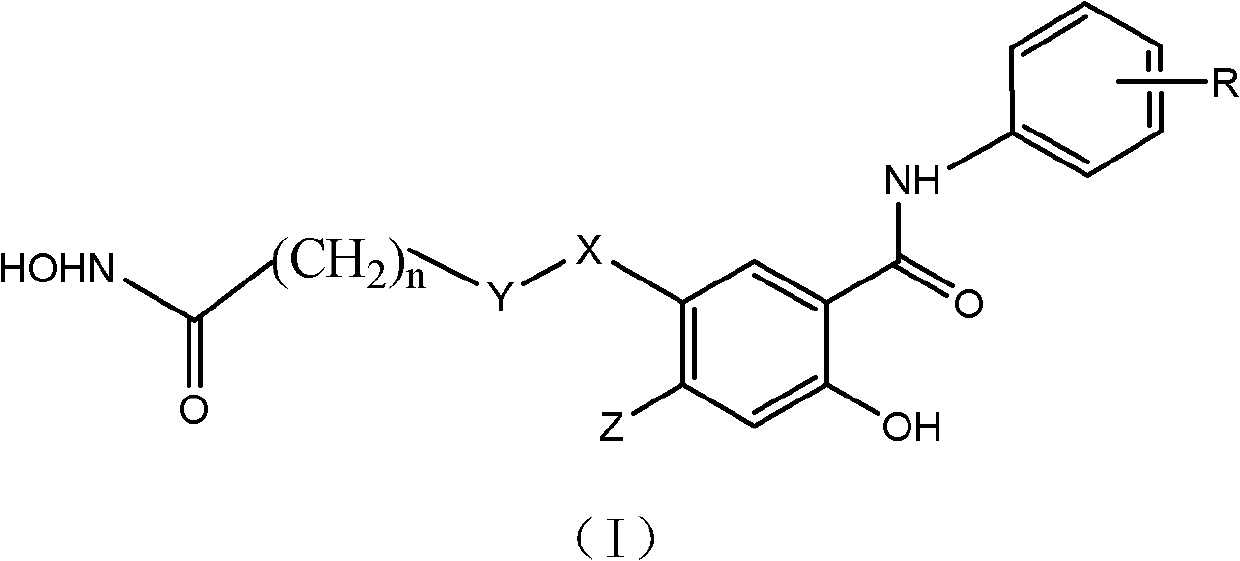
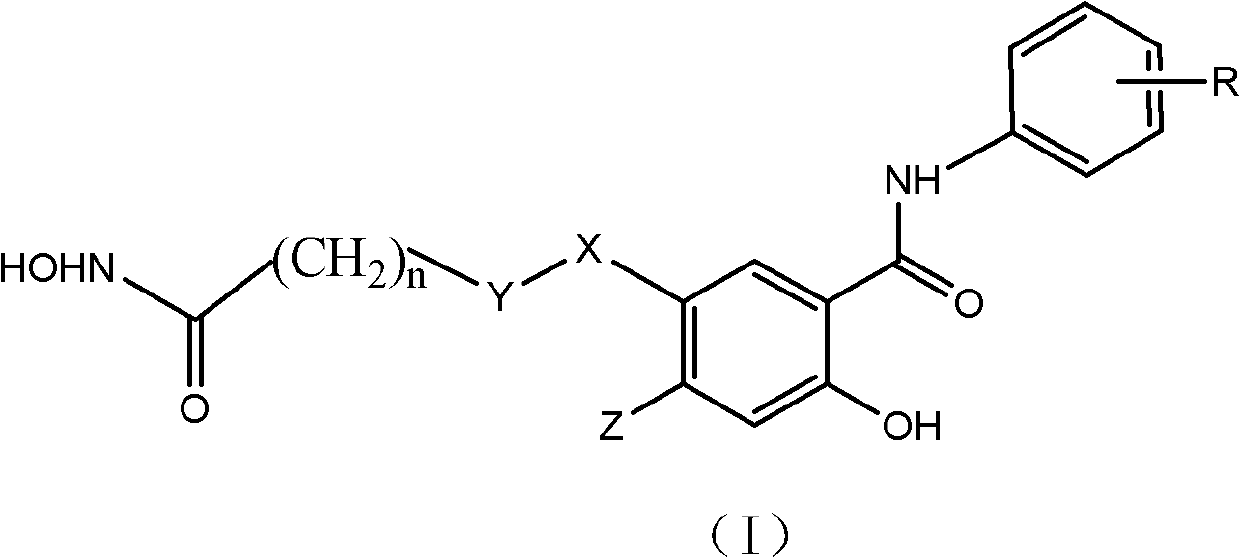
Salicylamide antitumor compound and its synthesis method and application
A technology of salicylamide and a synthesis method, which is applied in the field of antitumor drugs, can solve the problems of easy drug resistance and low efficacy of drugs, and achieve the effects of inhibiting tumor cell proliferation activity, simple structure and easy synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of 2-hydroxy-5-(7-hydroxyamino-7-oxoheptanyl)-N-phenylbenzamide (1)
[0047] 1A. 5-Nitro-2-hydroxy-N-phenylbenzamide
[0048]Add 2.75g (15mmol) of 5-nitrosalicylic acid, 2.1g (22.5mmol) of aniline, 5.8ml (22.5mmol) of triphenyl phosphite, and 30ml of toluene into a 100ml round bottom flask. Nitrogen protection was used to reflux for 8 hours, the reaction system was cooled to room temperature, and a pale yellow solid was precipitated. Add 20ml of dichloromethane to the system, filter with suction, wash the filter cake with cold dichloromethane (5ml×2), recrystallize from absolute ethanol (20ml) to obtain 3.2g of yellow powder, yield 84%, mp: 219- 221°C. MS: 258.93
[0049] 1B. 5-Amino-2-hydroxy-N-phenylbenzamide
[0050] In a 250ml round bottom flask, add 3.1g (12mmol) of 5-nitro-2hydroxy-N-phenylbenzamide, ethanol (40ml), tetrahydrofuran (40ml), iron powder 6.7g (120mmol), glacial acetic acid (10ml ), the system was refluxed for 7h and cooled ...
Embodiment 2
[0056] Example 2 Preparation of 2-hydroxy-5-(7-hydroxyamino-7-oxoheptanyl)-N-(3-fluorophenyl)benzamide (2)
[0057] 2A.5-Nitro-2-hydroxy-N-(3-fluorophenyl)benzamide
[0058] Same as the synthesis of compound 1A in Example 1, reflux in toluene with 2.38g (13mmol) of 5-nitrosalicylic acid, 1.11g (10mmol) of 3-fluoroaniline, and 3.4ml of triphenyl phosphite to obtain compound 2A, light yellow 2.6 g of white needle crystals, 94% yield, mp: 208-210°C. MS: 276.
[0059] 2B. 5-Amino-2-hydroxy-N-(3-fluorophenyl)benzamide
[0060] Similar to the synthesis of compound 1B in Example 1, using 1.95 g (7 mmol) of compound 2A as a raw material, ethanol, tetrahydrofuran, glacial acetic acid and iron powder were refluxed to obtain the target compound 2B, a gray solid, and the reaction was quantitatively completed. used directly in the next reaction.
[0061] 2C.2-Hydroxy-5-(7-ethoxy-7-oxoheptanyl)-N-(3-fluorophenyl)benzamide
[0062] Same as the synthesis of compound 1C in Example 1, the ...
Embodiment 3
[0065] Example 3 Preparation of 2-hydroxy-5-(7-hydroxyamino-7-oxoheptanyl)-N-(3,4-difluorophenyl)benzamide (3)
[0066] 3A. 5-Nitro-2-hydroxy-N-(3,4-difluorophenyl)benzamide
[0067] With the synthesis of Compound 1A in Example 1, 2.38g (13mmol) of 5-nitrosalicylic acid, 1.29g (10mmol) of 3,4-difluoroaniline, and 3.4ml of triphenyl phosphite were refluxed in toluene to obtain the target Compound 3A, 2.7 g of yellow-green needle crystals, yield 93%, mp: 257-258°C. MS: 294.
[0068] 3B. 5-Amino-2-hydroxy-N-(3,4-difluorophenyl)benzamide
[0069] Same as the synthesis of compound 1B in Example 1, using compound 3A 2.0g (6.8mmol) as raw material, ethanol, tetrahydrofuran, glacial acetic acid and iron powder were refluxed to obtain target compound 3B, off-white solid 1.62g, yield 90%, directly used in Next reaction.
[0070] 3C.2-Hydroxy-5-(7-ethoxy-7-oxoheptanoyl)-N-(3,4-difluorophenyl)benzamide
[0071] Same as the synthesis of compound 1C in Example 1, the compound 3B obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com