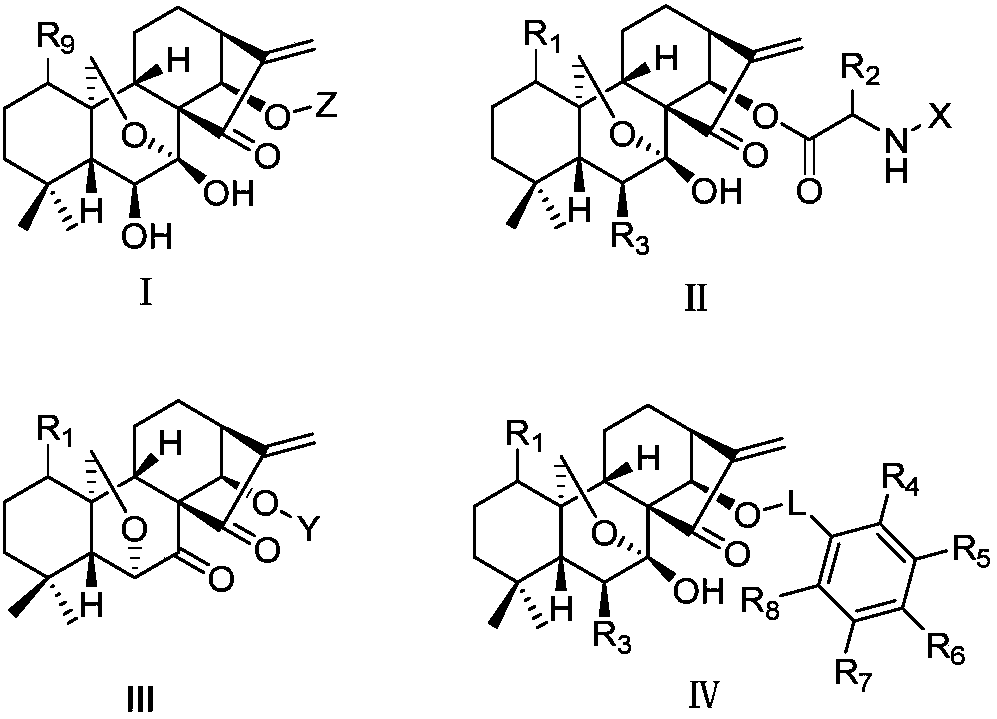
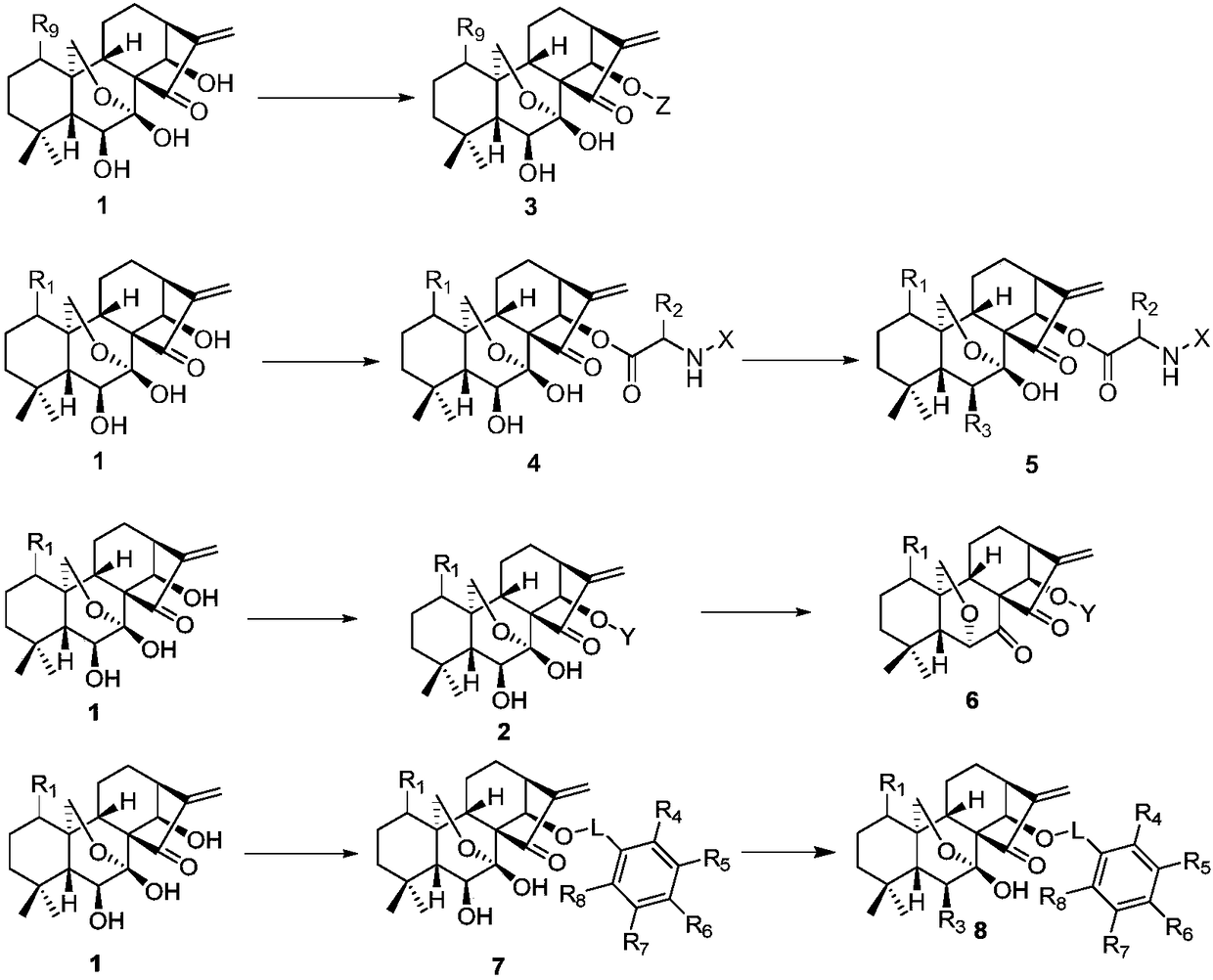
Rubescensin A derivatives and preparation method and applications thereof
A technology of oridonin A and its derivatives, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., and can solve the problem of not greatly improving antitumor activity, water solubility, and reducing toxicity. Comparing the selectivity difference of derivatives and restricting the development of Rubescensine A to achieve the effects of increasing bioavailability, reducing drug dosage and improving anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117]
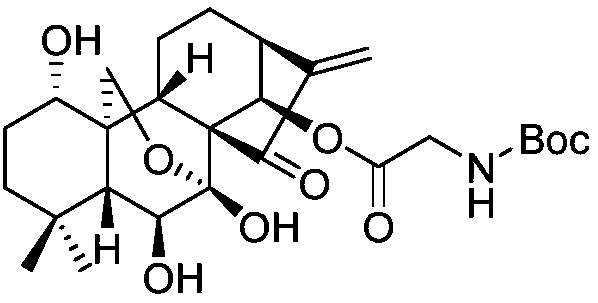
[0118] Oridonin (100mg, 0.27mmol), N-Boc-glycine (48mg, 0.27mmol), EDCI (157mg, 0.82mmol) and DMAP (101mg, 0.82mmol) were added to a 50ml eggplant-type bottle, under nitrogen Under protective conditions, 10 ml of dried dichloromethane was added, reacted in an ice bath for 1 h, then stirred and reacted overnight at room temperature, and TLC detected that the reaction was complete. The reaction solution was transferred to a separatory funnel, washed twice with 1M dilute hydrochloric acid, and the organic phase was taken, dried over anhydrous magnesium sulfate, and silica gel column chromatography to obtain a white solid.
[0119] ent-1α-hydroxy-6β,7β-dihydroxy-[14β-O-(Boc-L-glycine)]-15-oxo-7,20-oxo-16-kaurene 1 HNMR (500MHz, CDCl 3 )δ6.20(d, J=10.7Hz, 1H), 6.12(s, 1H), 5.95(s, 1H), 5.50(s, 1H), 5.37(t, J=5.2Hz, 1H), 4.52( s,1H),4.27(d,J=10.3Hz,1H),4.02(d,J=10.3Hz,1H),3.79(ddd,J=23.2,18.2,5.7Hz,2H),3.69(dd,J =10.8,7.0Hz,1H),3.44(dd,J=11.3,5.5Hz,1H),3.11(d,J=9.7Hz,...
Embodiment 2
[0121]
[0122] ent-1α-hydroxy-6β,7β-dihydroxy-[14β-O-(Boc-L-leucine)]-15-oxo-7,20-oxo-16-kaurene. Referring to the synthetic method of Example 1. 1 H NMR (500MHz, CDCl 3 )δ6.10(d, J=12.4Hz, 1H), 5.87(s, 1H), 5.47(s, 1H), 4.90(d, J=7.6Hz, 1H), 4.28(d, J=10.4Hz, 1H), 4.23(s, 1H), 4.18(s, 1H), 4.05(d, J=10.4Hz, 1H), 3.73(dd, J=10.6, 6.9Hz, 1H), 3.51–3.44(m, 1H ),3.12(d,J=9.8Hz,1H),2.63–2.54(m,1H),2.31–2.18(m,1H),1.99–1.90(m,1H),1.75(dt,J=20.2,6.4 Hz,1H),1.72–1.63(m,1H),1.63–1.52(m,2H),1.48–1.42(m,1H),1.38(s,4H),1.29–1.22(m,1H),1.10( s,3H),0.87(t,J=5.9Hz,3H). 13 C NMR (126MHz, CDCl 3 )δ206.25,171.84,155.28,149.89,119.99,96.01,79.96,77.24,76.52,74.40,73.37,63.38,62.01,59.51,54.71,52.52,41.30,40.89,38.70,33.70,32.62,30.49,29.89,28.23,24.77 ,22.71,21.94,21.73,19.84.HRESIMS m / z 578.3324[M+H] + .
Embodiment 3
[0124]
[0125] ent-1α-hydroxy-6β,7β-dihydroxy-[14β-O-(Fmoc-L-phenylalanine)]-15-oxo-7,20-oxo-16-kaurene. Referring to the synthetic method of Example 1. 1 H NMR (500MHz, CDCl 3 )δ7.76(d, J=7.4Hz, 2H), 7.55(d, J=7.3Hz, 2H), 7.40(t, J=7.4Hz, 2H), 7.32(t, J=7.4Hz, 2H) ,7.24(d,J=9.6Hz,2H),7.12(d,J=6.9Hz,2H),6.17(d,J=10.9Hz,1H),6.07(s,1H),5.95(s,1H) ,5.51–5.45(m,1H),5.40(s,1H),4.53(dd,J=13.4,6.6Hz,1H),4.37(dd,J=10.2,7.5Hz,1H),4.32(s,1H ),4.30–4.23(m,2H),4.17(t,J=7.0Hz,1H),4.03(d,J=10.3Hz,1H),3.72(dd,J=10.8,7.1Hz,1H),3.41 (d,J=5.9Hz,1H),3.12(dd,J=13.9,6.5Hz,1H),2.99(dd,J=13.8,6.3Hz,1H),2.93(d,J=9.7Hz,1H) ,2.52(dt,J=13.6,8.9Hz,1H),2.17(dt,J=21.5,13.4Hz,1H),1.92(s,1H),1.87(dd,J=12.9,5.3Hz,1H), 1.77–1.68(m,1H),1.63(d,J=3.5Hz,2H),1.60–1.48(m,2H),1.42(d,J=13.5Hz,1H),1.08(d,J=13.7Hz ,6H). 13 C NMR (126MHz, CDCl 3 )δ206.20,170.35,155.67,149.86,143.83,143.75,141.24,135.93,129.38,128.56,128.51,127.69,127.13,127.10,127.02,125.22,125.15,120.22,119.93,95.97,75.89,74.71,73.41,67.14,6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com