5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives and preparation method and application thereof
A technology of tert-butylthiazole and chlorobenzyl, applied in the field of 5--4-tert-butylthiazole derivatives and its preparation, can solve the problems of thiazole derivatives preparation and anti-tumor activity without research reports
Inactive Publication Date: 2010-09-29
HUNAN UNIV
View PDF2 Cites 19 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
There is no research report on the preparation and antitumor activity of 5-(4-chlorobenzyl)-4-tert-butylthiazole derivative (I)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Login to View More
Abstract
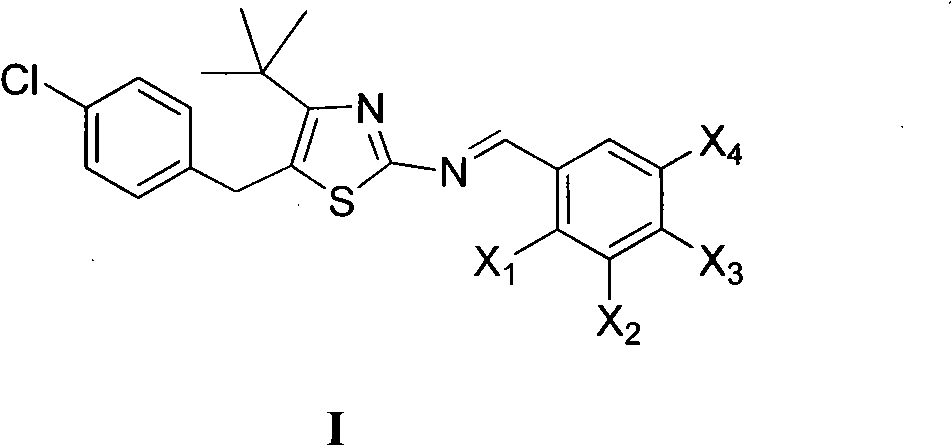
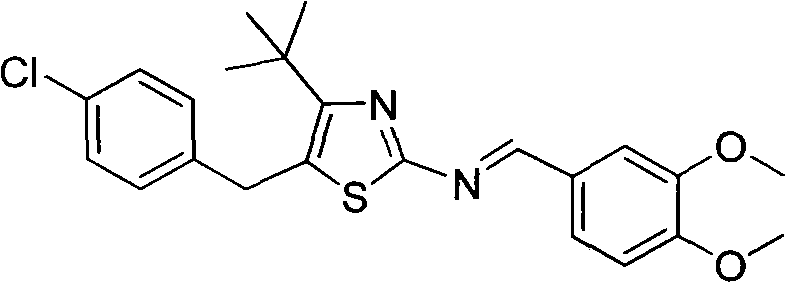
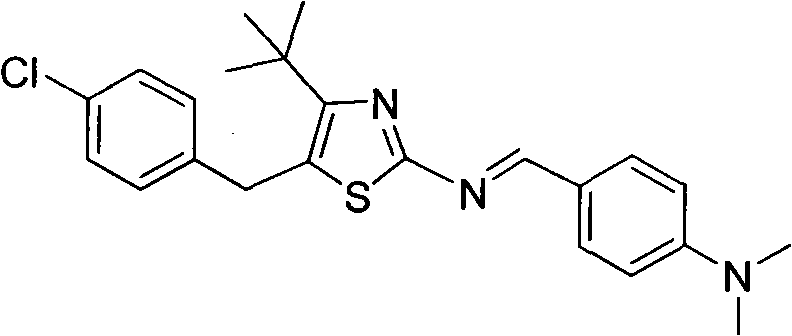
The invention discloses 5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives (I), which has the following structural formula as shown in the specification of the invention, wherein X1 in a formula I is selected from hydrogen, hydroxyl group, methoxy group, nitro group, amino group and chlorine; X2 is selected from hydrogen, nitro group, amino group, methoxy group, chlorine, bromine and iodine; X3 is selected from hydrogen, methyl group, ethyl group, nitro group, methoxy group, chlorine, bromine, amino group and dimethylamino group; and X4 is selected from hydrogen, nitro group, amino group, methoxy group, chlorine, bromine and iodine. The 5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives (I) have favorable inhibition activity on human cervical cancer cells, human liver cancer cells, human nasal and oral cancer cells and the like and can be used for preparing anti-tumor medicines.
Description
technical field The present invention relates to novel compounds and their preparation methods and applications, in particular to 5-(4-chlorobenzyl)-4-tert-butylthiazole derivatives and their preparation methods and applications. Background technique Among China's 1.3 billion people, one million people die of cancer every year, about 2 million need treatment, and about 1.3 million die. Cancer has become a major killer of human beings. Thiazole compounds have anticonvulsant, antiviral, antibacterial and insecticidal effects. Shao Ling et al. described 4-aryl-5-triazolylthiazole-2-imine compounds, and the results of biological activity assays showed that some of the compounds had bactericidal activity against P. ). Michael etc. have described 2-aminothiazole class breast cancer drug (Bioorg&Med.Chem.2004,12,1029); Acta Sinica, 2006, 41:727). Lin et al. described that Schiff bases have selective inhibitory effect on COX-2 (Bioorg & Med Chem, 2008, 16(5):2697). He Daohang ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D277/42A61K31/426A61P35/00
Inventor 胡艾希覃智夏曙向建南李珺姊
Owner HUNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com