Pyrazolopyrimidine-type compound, and preparation method and application thereof
A pyrazolopyrimidine and azolopyrimidine technology, which is applied in the field of pyrazolopyrimidine compounds and their preparation, can solve the problems of poor therapeutic effect and uniform drug resistance of targeted therapy drugs, and achieve low cost and high synthesis efficiency. The effect of short route and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
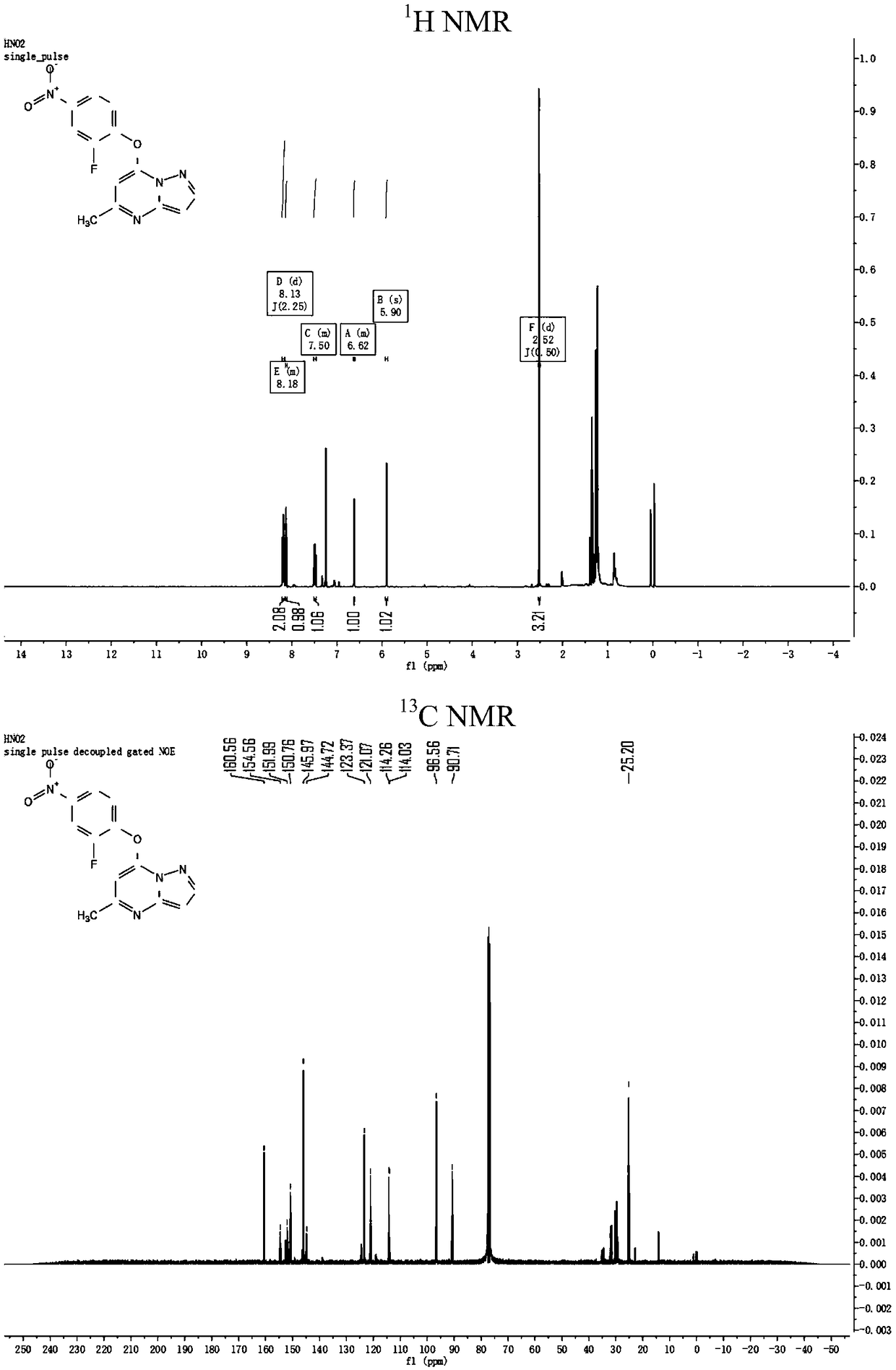
[0057] An embodiment of the preparation method of pyrazolopyrimidine compounds of the present invention, the compound name is 7-[2-fluoro-4-(3,5,6-trimethylpyrazinecarboxamido)phenoxy] -5-methylpyrazolo[1,5-a]pyrimidine, the R group is 3,5,6-trimethylpyrazine, and the preparation method comprises the following steps:
[0058] (1) Synthesis of intermediate 5-methyl-7-hydroxy-pyrazolo[1,5-a]pyrimidine
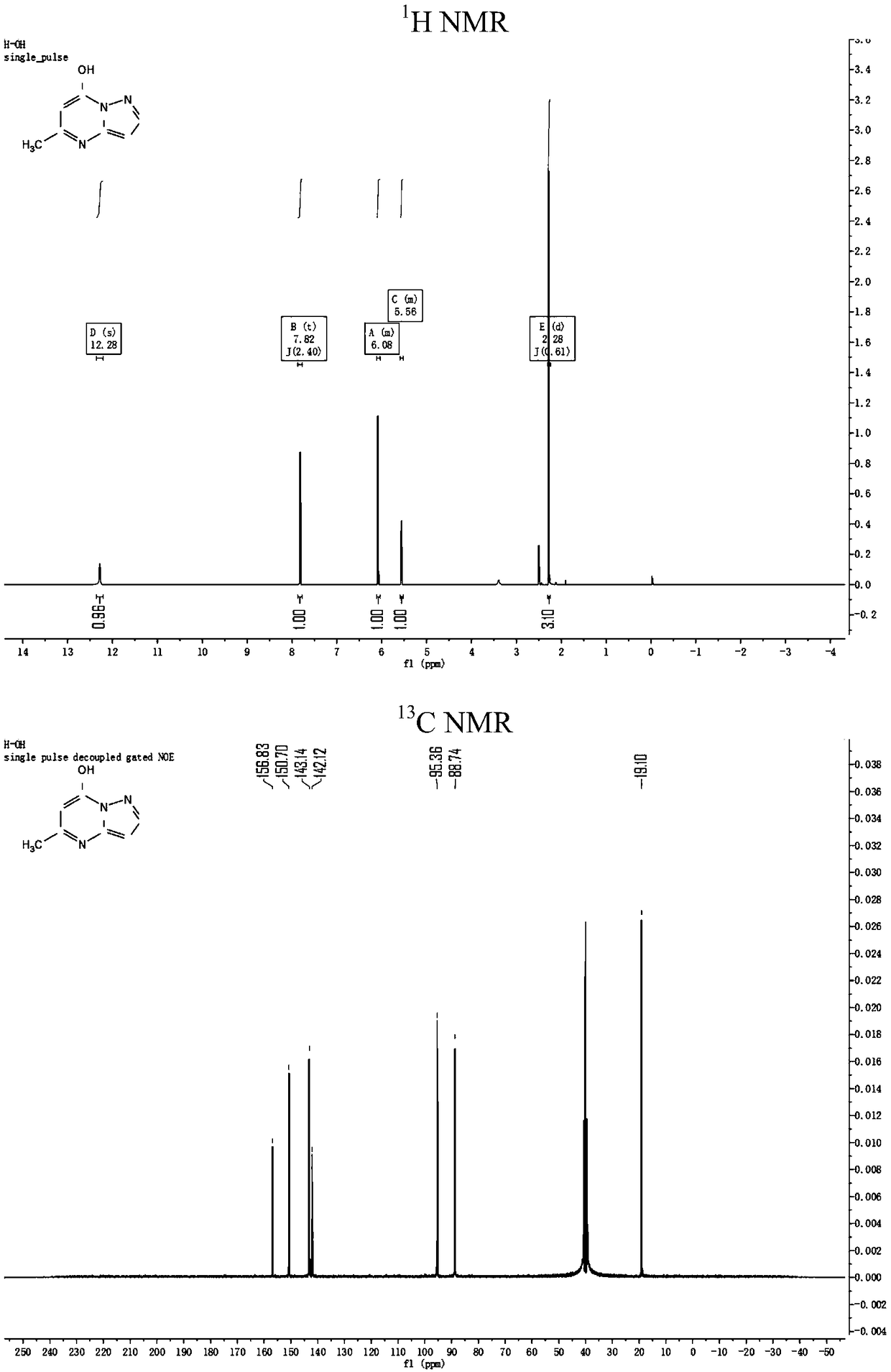
[0059] Weigh 4.860g (58.000mmol) of 3-aminopyrazole and 7.541g (58.000mmol) of ethyl acetoacetate into a round bottom flask, add 20mL of glacial acetic acid as the reaction solvent, heat and stir, and react at 120°C for 1.5h. TLC detection; after the reaction is finished, cool, filter with suction, and wash the upper precipitate with absolute ethanol to obtain 6.103 g of a white solid, which is the intermediate 5-methyl-7-hydroxyl-pyrazolo[1,5-a ] pyrimidine ( 1 H NMR and 13 C NMR spectrum as figure 1 Shown), productive rate 70%;
[0060] 1 H NMR (400MHz, DMSO-d 6 )δ12.28(...
Embodiment 2
[0080] An embodiment of the preparation method of pyrazolopyrimidine compounds of the present invention, the compound name is 7-[2-fluoro-4-(pyrazinecarboxamido)phenoxy]-5-methylpyrazolo[ 1,5-a] pyrimidine, R base is 2-pyrazinyl, and the preparation method comprises the following steps:
[0081] (1) Synthesis of intermediate 5-methyl-7-hydroxy-pyrazolo[1,5-a]pyrimidine
[0082] Weigh 69.600mmol of 3-aminopyrazole and 58.000mmol of ethyl acetoacetate into a round bottom flask, add 20mL of glacial acetic acid as a reaction solvent, heat and stir, react at 100°C for 2.5h, and detect by TLC; after the reaction, cool , filtered with suction, and washed the upper precipitate with absolute ethanol to obtain 6.103 g of a white solid, which is the intermediate 5-methyl-7-hydroxy-pyrazolo[1,5-a]pyrimidine, with a yield of 70%;
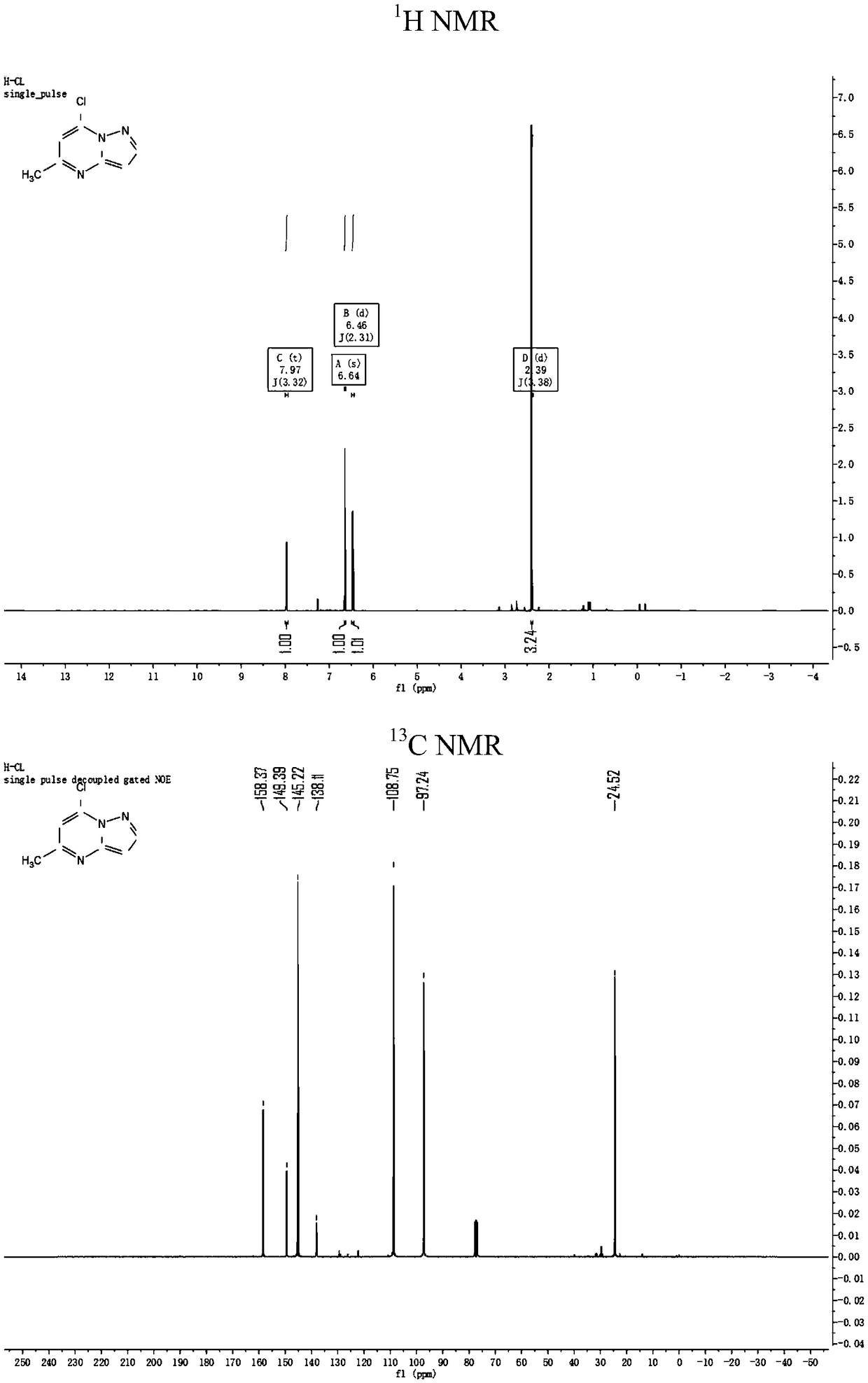
[0083] (2) Synthesis of intermediate 5-methyl-7-chloro-pyrazolo[1,5-a]pyrimidine
[0084] Weigh 2.500g (16.800mmol) of 5-methyl-7-hydroxy-pyrazolo[1,5-a]pyrim...
Embodiment 3
[0094] An embodiment of the preparation method of pyrazolopyrimidine compounds of the present invention, the compound name is 7-[2-fluoro-4-(5-pyrimidinecarboxamido)phenoxy]-5-methylpyrazolo [1,5-a]pyrimidine, the R group is 5-pyrimidinyl, and the preparation method comprises the following steps:
[0095] (1) Synthesis of intermediate 5-methyl-7-hydroxy-pyrazolo[1,5-a]pyrimidine
[0096] Weigh 63.800mmol of 3-aminopyrazole and 58.000mmol of ethyl acetoacetate into a round bottom flask, add 20mL of glacial acetic acid as a reaction solvent, heat and stir, react at 140°C for 1.8h, and detect by TLC; after the reaction, cool , filtered with suction, and washed the upper precipitate with absolute ethanol to obtain 6.103 g of a white solid, which is the intermediate 5-methyl-7-hydroxy-pyrazolo[1,5-a]pyrimidine, with a yield of 70%;
[0097] (2) Synthesis of intermediate 5-methyl-7-chloro-pyrazolo[1,5-a]pyrimidine
[0098] Weigh 2.500g (16.800mmol) of 5-methyl-7-hydroxy-pyrazolo[1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com