Applicationof A-nor-5 alpha-androstane compounds in preparation of malignant tumor resistant medicaments
A malignant tumor, androstane technology, applied in the direction of antitumor drugs, steroids, drug combinations, etc., can solve the problems of toxicity, lack of selective tumor inhibition, damage, etc., achieve low drug toxicity, inhibit malignant tumor cells, etc. Good growth and healing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
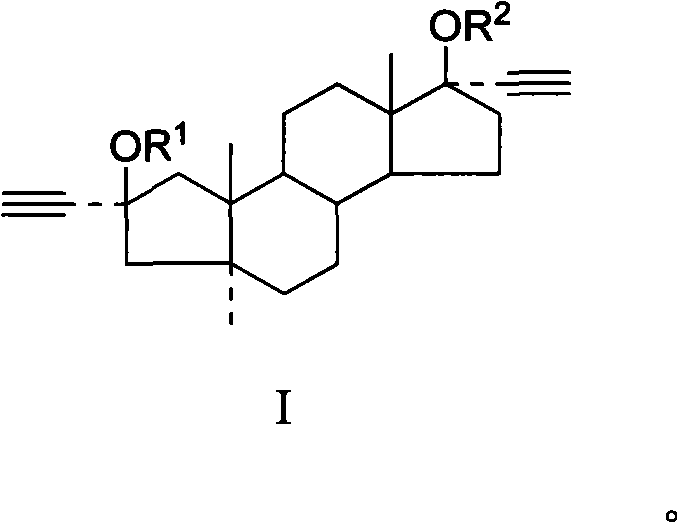
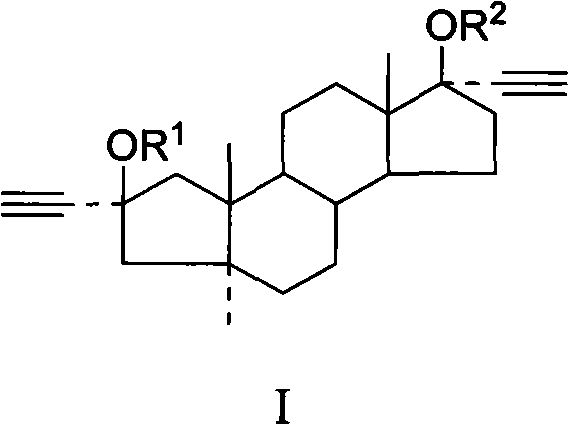
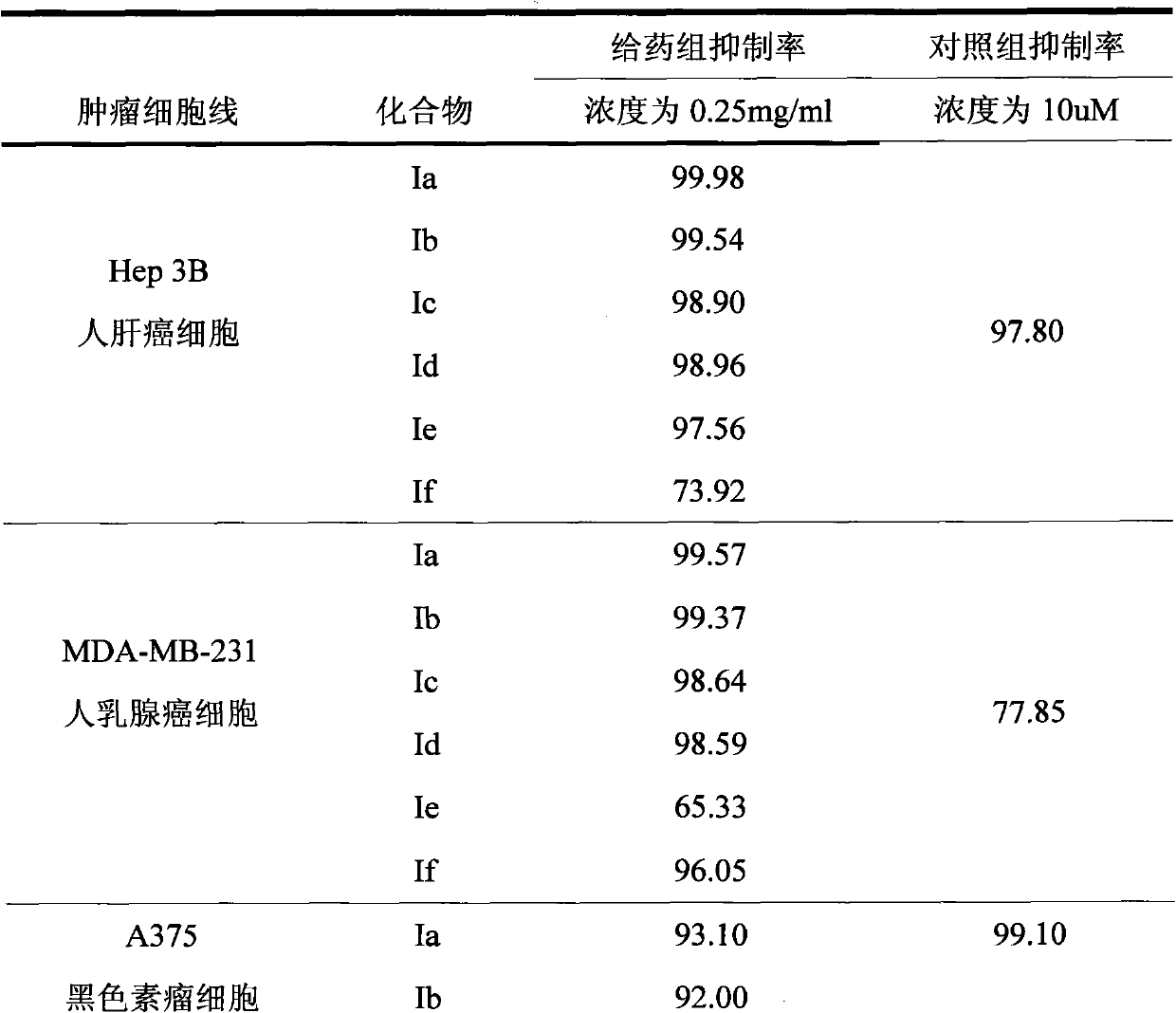
[0043] Example 1 Preparation of 2α, 17α-bisethynyl-A-abortion-5α-androstane-2β, 17β-dihydroxy compound (Ia)
[0044] Suspend 30g of KOH powder in 60ml of THF, cool to below 5°C and pass through acetylene until no absorption, then dissolve 10g of A-anorsandrostane-2,17-dione in 100ml of THF, add dropwise to the flask, pass through acetylene until thin The raw material disappeared in layer chromatography, slowly added 18ml of water dropwise under stirring, stirred for 30 minutes, separated the lower aqueous layer, concentrated the organic layer to dryness under reduced pressure, added 100mL of ethyl acetate to dissolve, washed with water until the pH was 7, and 5g of activated carbon Decolorize, filter, and concentrate the filtrate to dryness under reduced pressure. The viscous solid obtained is refluxed and dissolved in 150 mL of n-hexane-benzene mixed solvent with a volume ratio of 1:1. After cooling, crystals are precipitated, filtered, and the filter cake is dried to obtain 2...
Embodiment 2
[0045] Example 2 Preparation of 2α, 17α-bisethynyl-A-abortion-5α-androstane-2β, 17β-dihydroxydiacetate (Ib)
[0046] Dissolve 2g of Ia in 20mL of pyridine, add 20mL of acetic anhydride and 0.1g of sodium acetate, reflux for 10 hours, cool to 20°C after the reaction is complete, slowly add 30mL of methanol dropwise, add 20mL of saturated saline, and use ethyl acetate (15mL*3 ), combined organic layers, washed with water until the pH was 7, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure, and the obtained solid was dissolved in 20 mL of methanol at 65 ° C, cooled to 20 ° C and left for 24 hours, and the solid was precipitated. Filter and dry the filter cake to obtain 1.3g of 2α, 17α-diethynyl-A-carbocarb-5α-androstane-2β, 17β-bishydroxydiacetate (Ib), mp190-191°C, [α ] D 25 -39° (C=1, CHCl 3 ).
Embodiment 3
[0047] Example 3 Preparation of 2α, 17α-bisethynyl-A-abort-5α-androstane-2β, 17β-dihydroxydipropionate (Ic)
[0048] Dissolve 2g of Ia in 20mL of pyridine, add 20mL of propionic anhydride and 0.1g of sodium acetate, reflux for 10 hours, cool to 20°C after the reaction is complete, slowly add 30mL of methanol dropwise, add 20mL of saturated saline, and use ethyl acetate (15mL*3 ), combined the organic layers, washed with water until the pH was 7, dried over anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness under reduced pressure. Crystals were filtered and the filter cake was dried to obtain 2α, 17α-bisethynyl-A-aborb-5α-androstane-2β, 17β-dihydroxydipropionate (Ic) 2g, mp152-153°C, [ α] D 25 -32° (C=1, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com