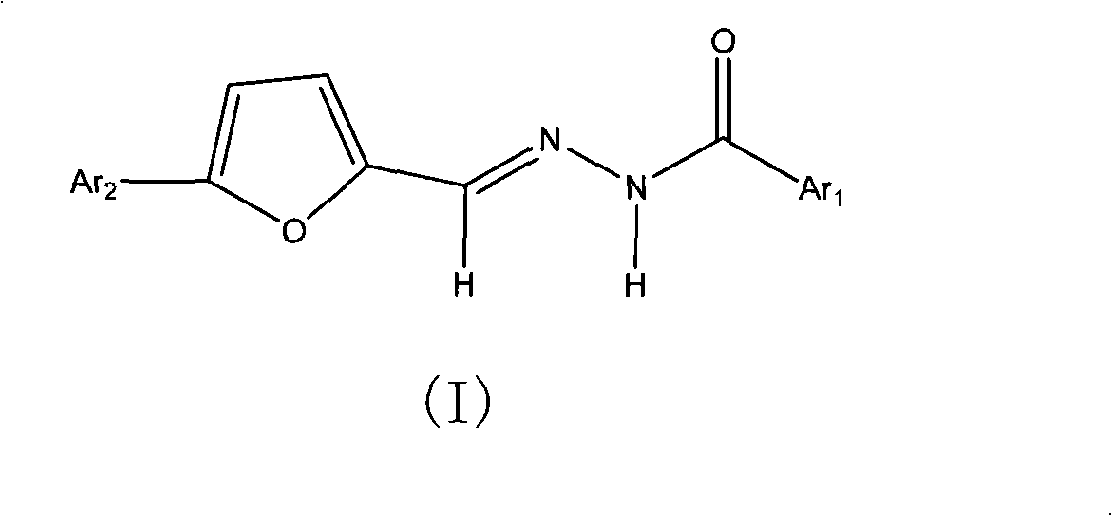
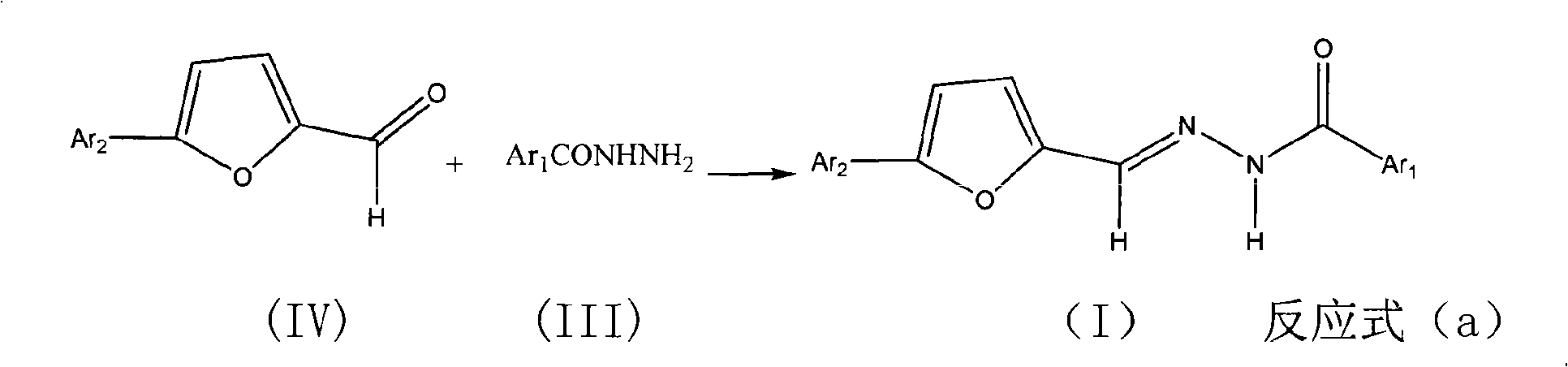
Benzoyl hydrazone compounds with antineoplastic activity
A technology of benzoyl hydrazone and compound, applied in the application field of anti-tumor growth inhibitor, can solve the problems such as unmentioned anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of o-hydroxybenzoic hydrazide
[0028]
[0029] Add 1.38g (0.01mol) of p-salicylic acid and 32g (0.1mol) of methanol into a 50ml three-necked flask, and slowly add 3.0ml of concentrated sulfuric acid while stirring. Heat to reflux for 8 hours, distill off excess methanol, filter the raffinate to remove unreacted salicylic acid, wash the filtrate repeatedly with water to weak acidity, distill under reduced pressure, collect fractions at 84°C / 0.093kPa to obtain 1.31g of light yellow liquid, yield 86.2%.
[0030] Add 1.31g (8.6mmol) light yellow liquid methyl salicylate and 0.1mol 50% hydrazine hydrate into 30ml absolute ethanol, reflux for 8h, let stand overnight, and precipitate a white solid. Filter and recrystallize with 50% ethanol to obtain 0.98g of salicylhydrazide with a yield of 75.0%. Melting point: 145-147°C.
Embodiment 2
[0031] Example 2: Preparation of 5-(2', 4'-difluorophenyl)-furan-2-carbaldehyde
[0032] Dissolve 12.9g (0.1mol) of 2,4-difluoroaniline in 10ml of water and 25ml of concentrated hydrochloric acid, add 25g of crushed ice, add 25ml of aqueous solution of 6.9g (0.1mol) of sodium nitrite dropwise below 5°C, stir for 10min, then add 9.6 25ml of acetone solution of g (0.1mol) furfural aldehyde and 3.0g of copper chloride were reacted at 25-30°C for 5h. Suction-filtered and dried, and recrystallized with 50% ethanol to obtain 7.46 g of 5-(2',4'-difluorophenyl)-furan-2-carbaldehyde, which appeared as a brown solid, m.p.: 118-119°C, yield 35.9 %.
Embodiment 3
[0033] Example 3: Preparation of N'-5-(2',4'-difluorophenyl)-2-furan-N-(2'-hydroxybenzoyl)hydrazone (I5)
[0034]
[0035] Add 30ml of absolute ethanol to a 50ml three-necked flask equipped with a thermometer, mix 1.04g (0.005mol) of 5-(2',4'-difluorophenyl)-furan-2-carbaldehyde with 0.76g (0.005mol) of water Salicylic hydrazide was added thereto, heated to reflux for 5h. After the reaction solution was cooled, a large amount of solids precipitated out. After filtration, a light yellow solid was obtained. After recrystallization from absolute ethanol, 1.43 g of light yellow needle-shaped crystals were obtained, with a yield of 83.6% and a melting point of 248-249°C.
[0036] 1 H-NMR (DMSO-d 6 ,δ H ppm): 6.98-7.05 (m, 3H, 2ArH+FuH), 7.13 (d, J=3.54Hz, 1H, FuH), 7.27 (td, J=8.45, 2.34Hz, 1H, ArH-Fu), 7.41- 7.48(m, 2H, ArH-Fu+ArH), 7.86-7.93(m, 2H, ArH-Fu+ArH), 8.42(s, 1H, CH=N), 11.80(s, 1H, OH), 11.87( s, 1H, NH). IR (cm -1 ): 3300, 3100, 2700, 1630, 1600, 1530, 1500,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com