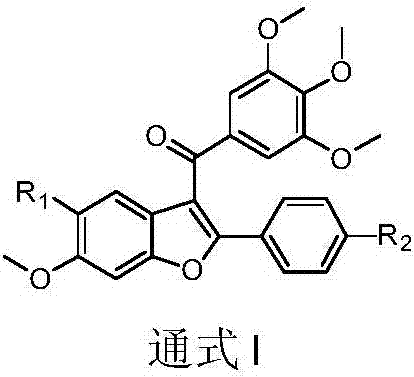
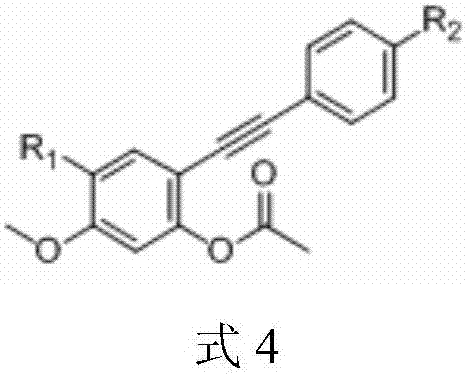
3-(3,4,5-trimethoxybenzoyl)-benzofuran microtubulin inhibitor as well as preparation method and use thereof
A technology of trimethoxybenzoyl and trimethoxybenzoyl chloride, applied in the field of medicinal chemistry, can solve the problems of low bioavailability, limited wide application, easy dissimilation, etc., achieve strong proliferation inhibitory activity and inhibit tubulin Aggregation, effect of excellent tubulin inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
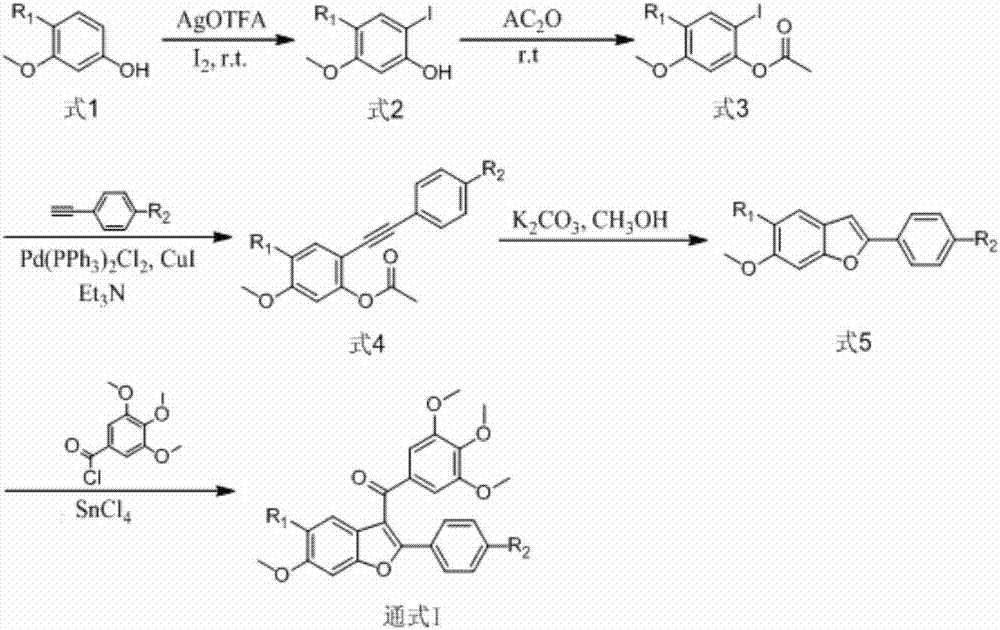
[0032] Embodiment 1: the preparation of structural formula 1 compound
[0033] (1) In a 250mL round bottom flask wrapped with tinfoil, I 2 (5.1 g, 20.0 mmol) was dissolved in chloroform (150 mL) and stirred over 1.5 hours. In addition, compound formula 1 (R 1 =H) (20.0 mmol) was placed in a 250 mL round bottom flask wrapped with tinfoil, and silver trifluoroacetate AgOTFA (4.5 g, 20.0 mmol) was added. The chloroform solution was slowly (about 2-3 hours) added to the flask wrapped in tin foil, and the reaction was stirred at room temperature for 24 hours to end. Filter and wash the residue with chloroform. then saturate with Na 2 S 2 o 3 solution, saturated NaHCO 3 solution, extracted with saturated brine, and the resulting organic phase was washed with anhydrous Na 2 SO 4 dry. Filter, carry out column chromatography separation (with CH 2 Cl 2 as eluent, column chromatography protected from light). Obtain product, 2-iodo-5-methoxyphenol (formula 2, R 1 = H). Whit...
Embodiment 2
[0044] Embodiment 2: the preparation of structural formula 2 compound
[0045] (1) The phenolic raw material is formula 1 (R 1 =F), step is the same as step (1) in embodiment 1. Obtain product 2-iodo-4-fluoro-5-methoxyphenol (formula 2, R 1 =F).
[0046]
[0047] White solid, yield 39.92%. 1 H NMR (500MHz, CDCl 3 )δ=7.31(d,J=10Hz,1H),6.67(d,J=7.5Hz,1H),5.13-5.12(m,1H),3.85(s,3H). 19F NMR (470MHz, CDCl 3 )δ=-143.08. 13 C NMR (125MHz, CDCl 3 )δ=151.73(d, J=2.5Hz), 149.2, 146.82(d, J=240.6Hz), 123.70(d, J=22.5Hz), 100.30, 70.97(d, J=7.5Hz), 56.27.ESI -HRMS(m / z): calculated for C 7 h 6 FIO 2 (M+H) + ,268.94301,found:268.94769.
[0048] (2) The steps are the same as the steps (2) in Example 1. Obtain product 2-iodo-4-fluoro-5-methoxyphenyl acetate (formula 3, R 1 =F).
[0049]
[0050] White crystals, yield 81.28%. 1 H NMR (500MHz, CDCl 3 )δ=7.47(d,J=10Hz,1H),6.74(d,J=7.5Hz,1H),3.85(s,3H),2.35(s,3H). 19 F NMR (470MHz, CDCl 3 )δ=-135.80. 13 C NMR (125MHz, ...
Embodiment 3
[0060] Embodiment 3: anti-proliferation experiment
[0061] 1. Experimental method
[0062] Cells with a live cell ratio of more than 90% were used for experiments. Cell Proliferation Inhibition Assay Using EnoGeneCell TM Counting Kit-8 (CCK-8) Cell Viability Detection Kit. Cells were digested, counted, and made into a cell suspension with a concentration of 1×105 / mL, and 100 μL of cell suspension was added to each well of a 96-well plate (1×104 cells per well); the 96-well plate was placed at 37°C for 5 %CO 2 Cultivate in an incubator for 24 hours; add 100 μL of corresponding drug-containing medium to each well, and set up a negative control group, a vehicle control group, and a positive control group at the same time, with 5 replicate wells in each group; place the 96-well plate at 37°C, 5% CO 2 After culturing in the incubator for 72 hours; add 10 μL of CCK-8 solution to each well, incubate the culture plate in the incubator for 4 hours, measure the OD value at 450 nm w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com