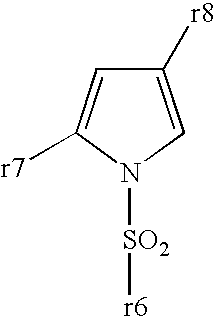
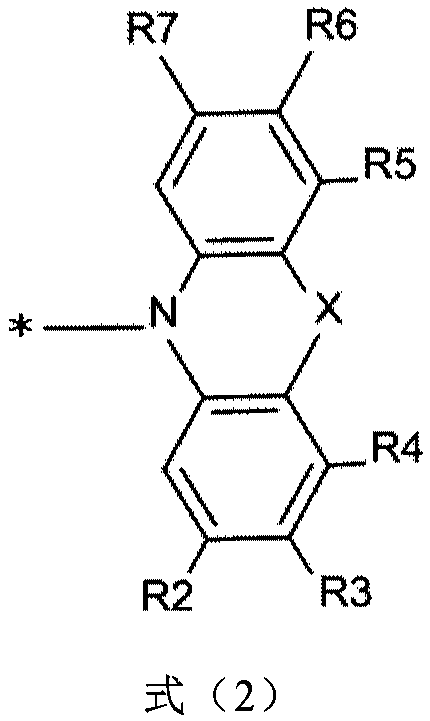
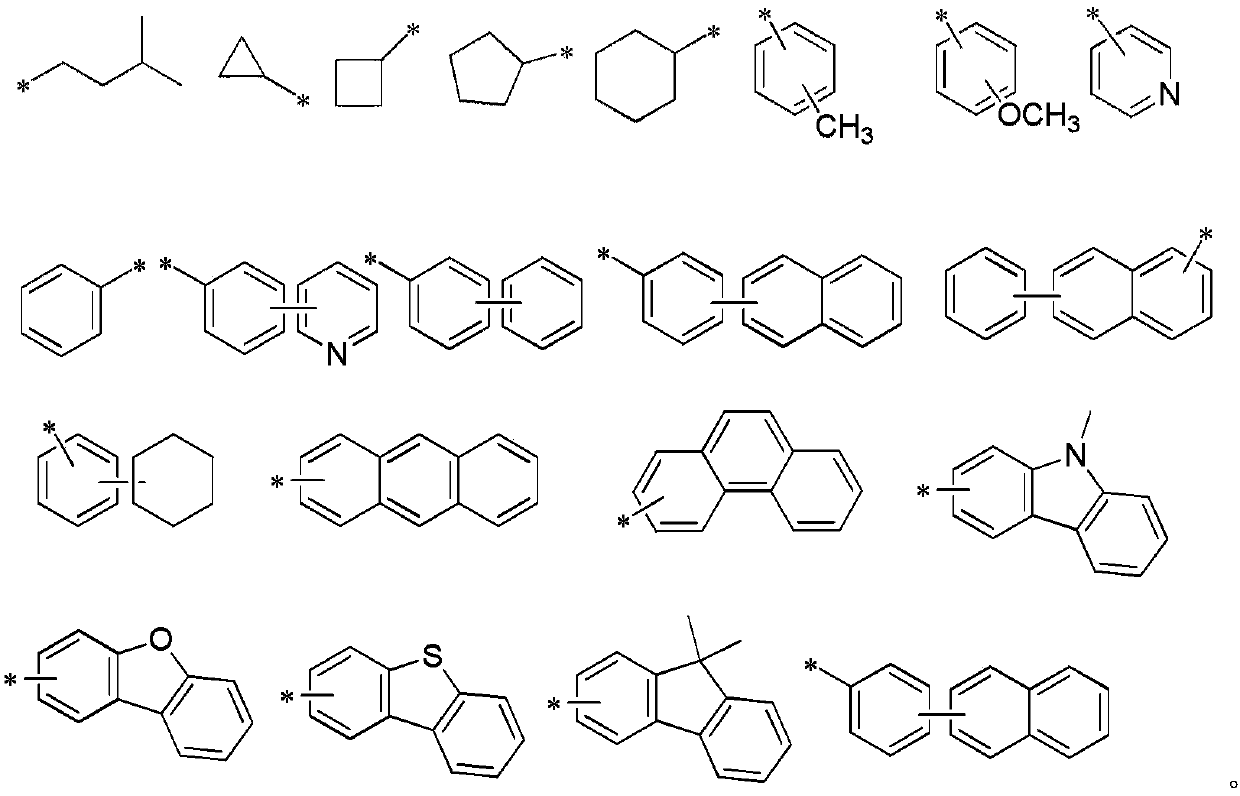
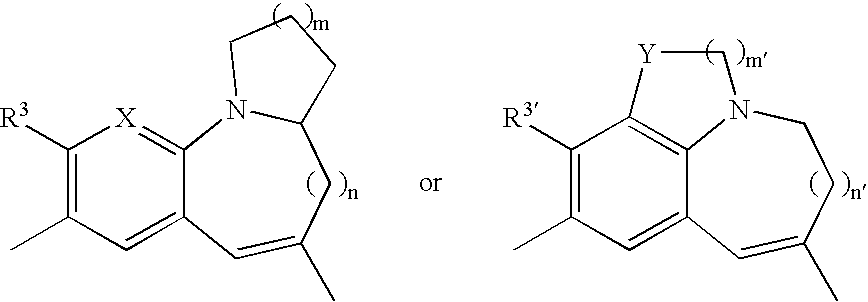
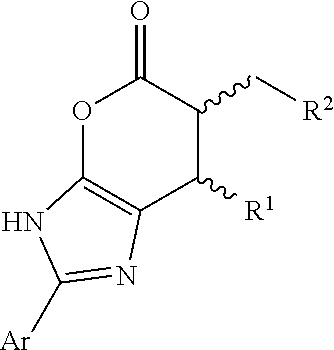
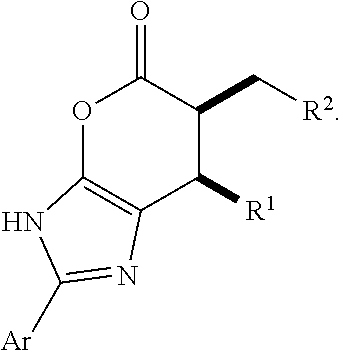
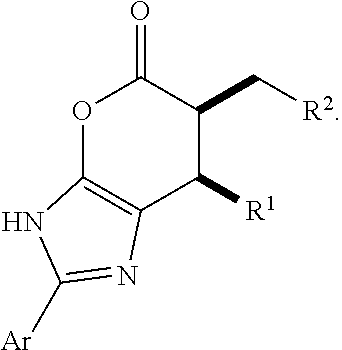
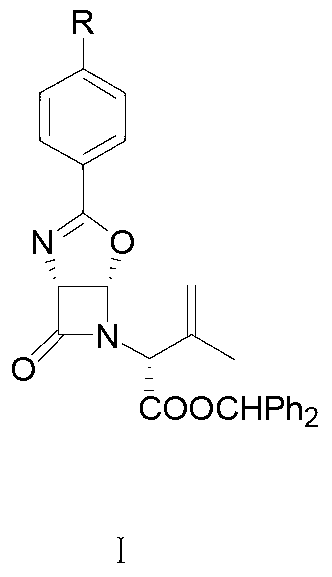
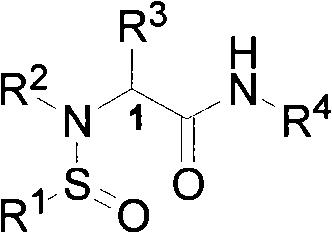
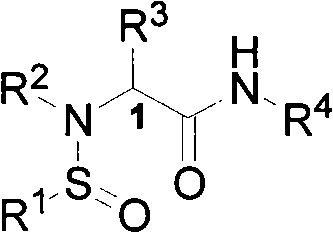
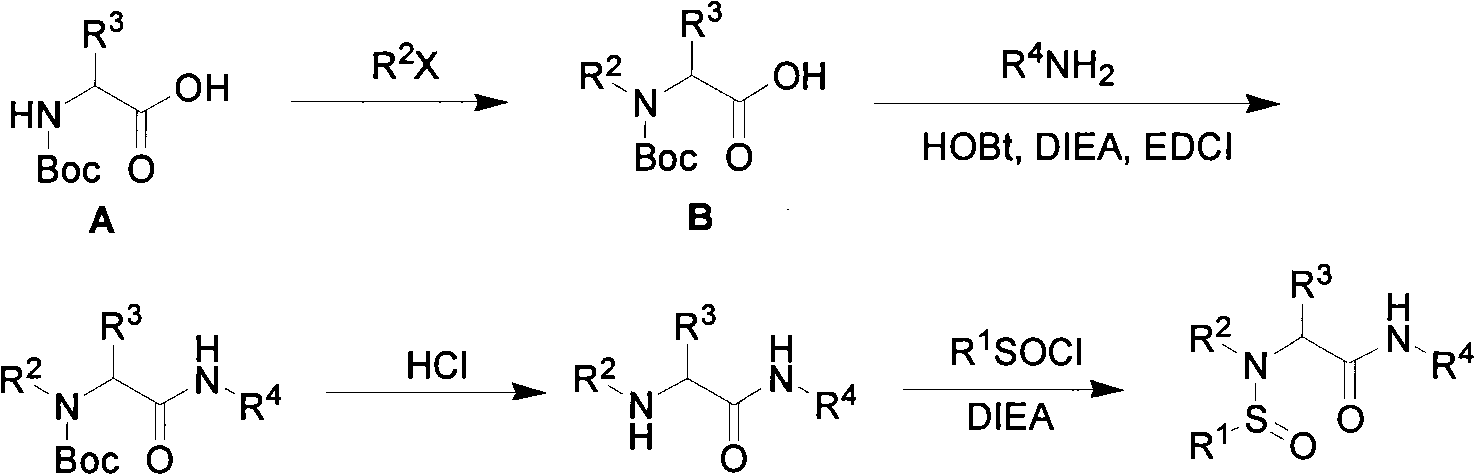
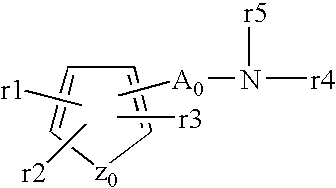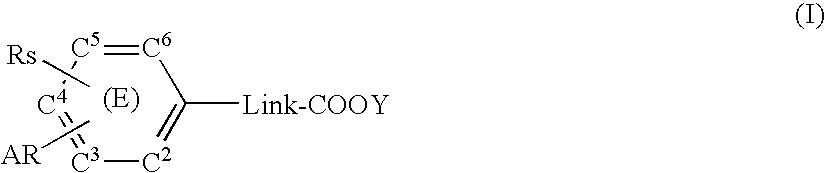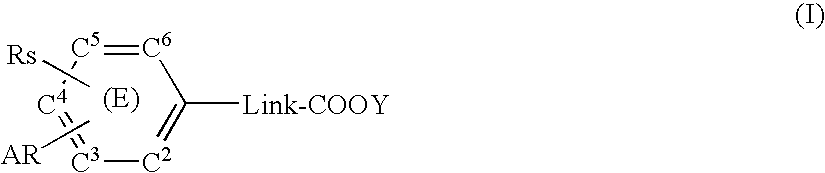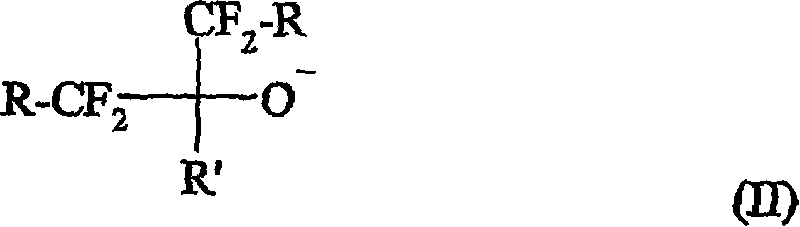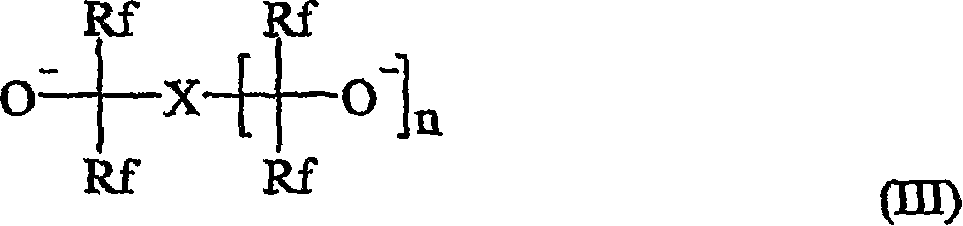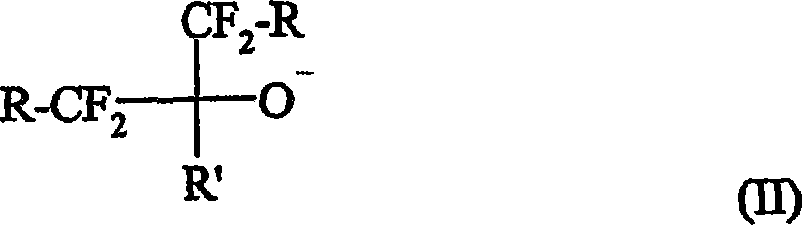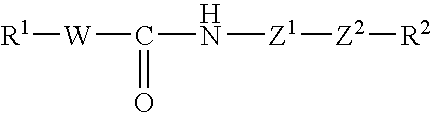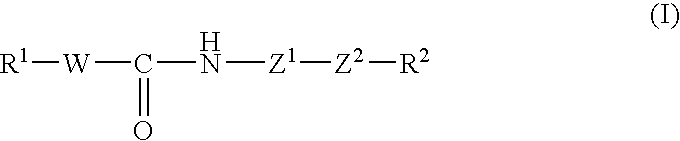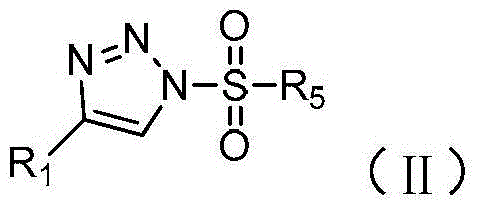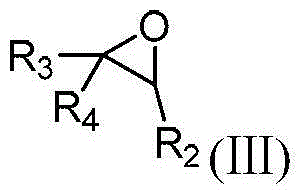Patents
Literature
163 results about "Annulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In organic chemistry annulation (from the Latin anellus for "little ring"; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule. Examples are the Robinson annulation, Danheiser annulation and certain cycloadditions. Annular molecules are constructed from side-on condensed cyclic segments, for example helicenes and acenes. In transannulation a bicyclic molecule is created by intramolecular carbon-carbon bond formation in a large monocyclic ring. An example is the samarium(II) iodide induced ketone - alkene cyclization of 5-methylenecyclooctanone which proceeds through a ketyl intermediate...
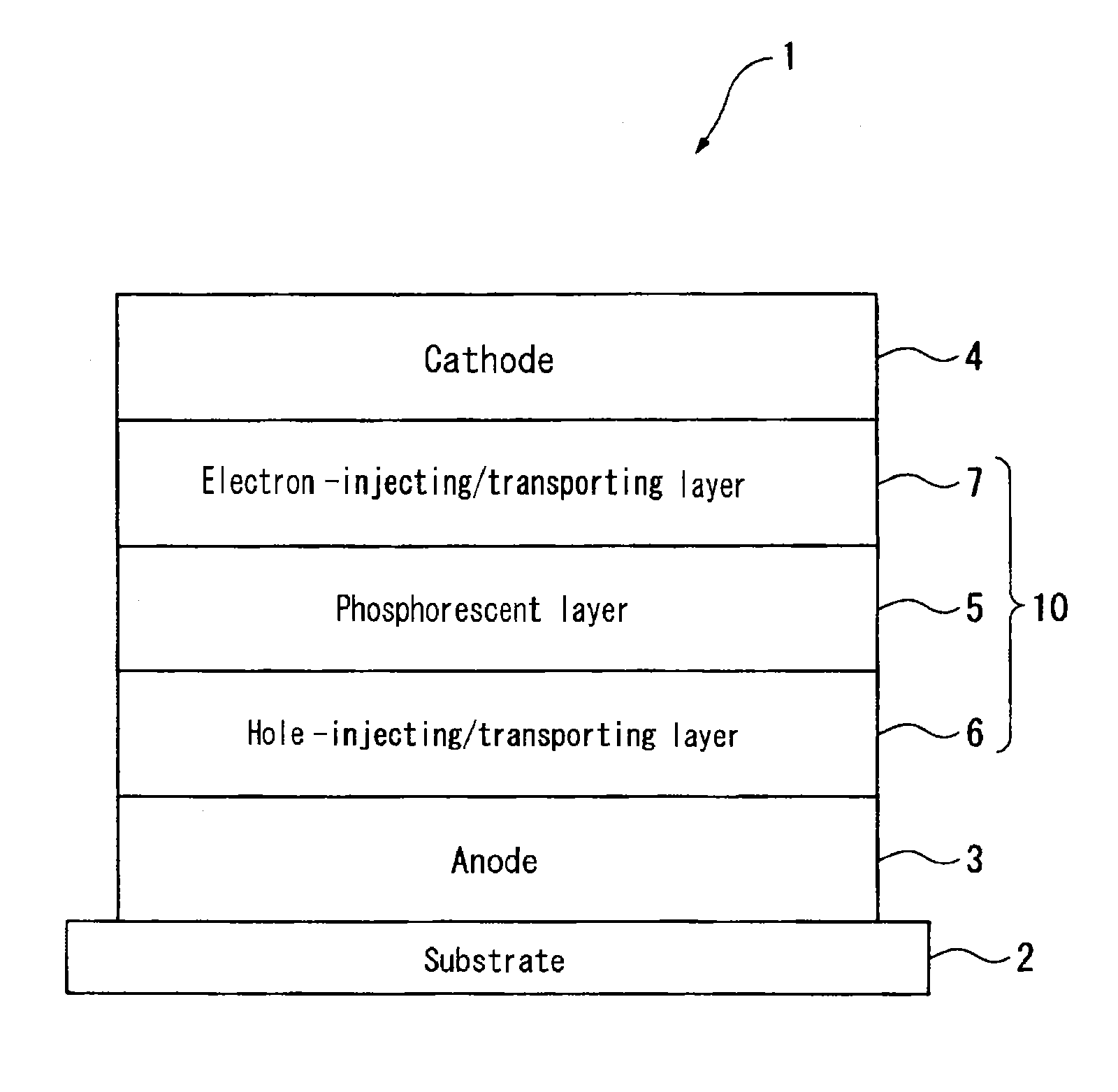
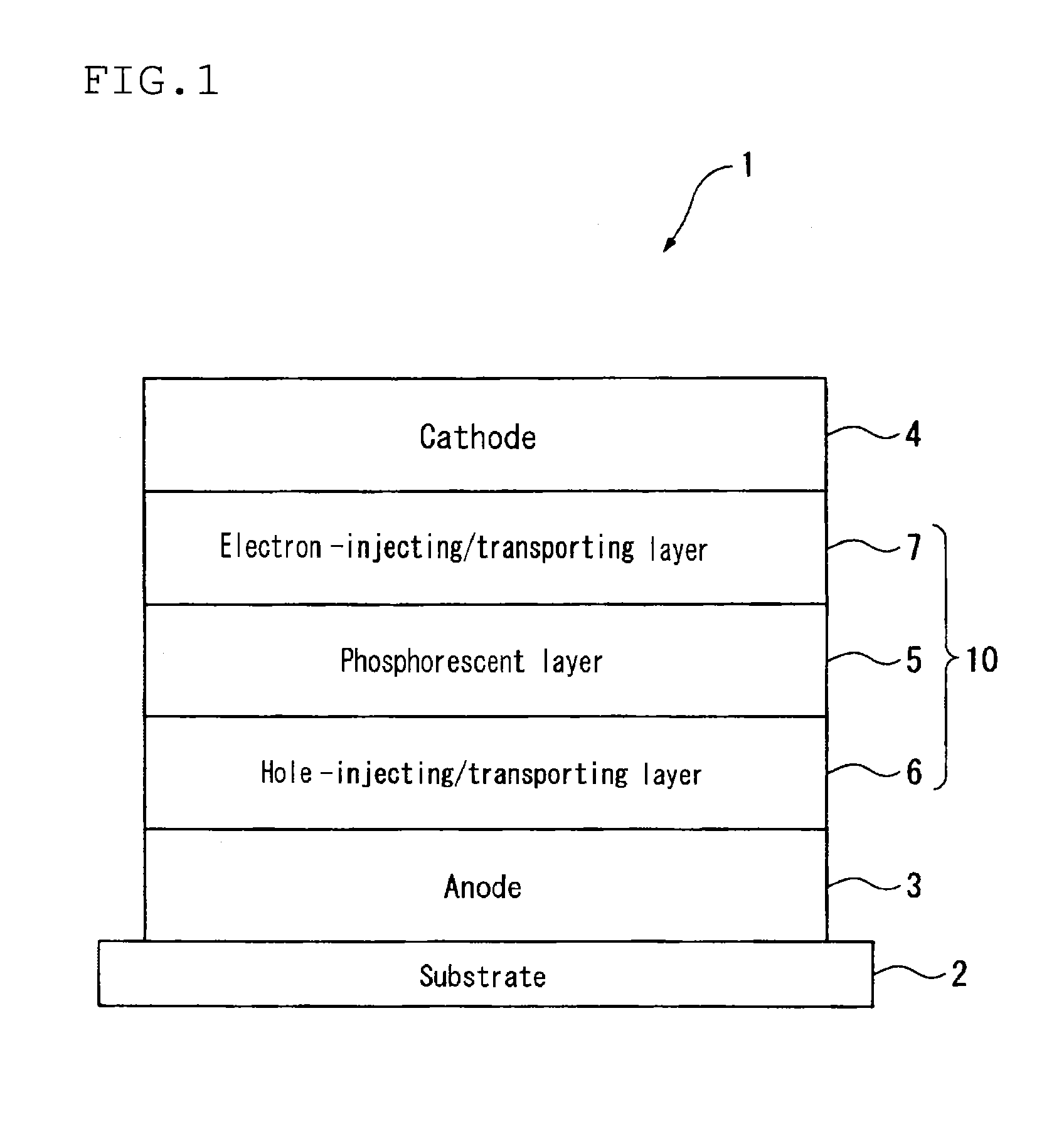
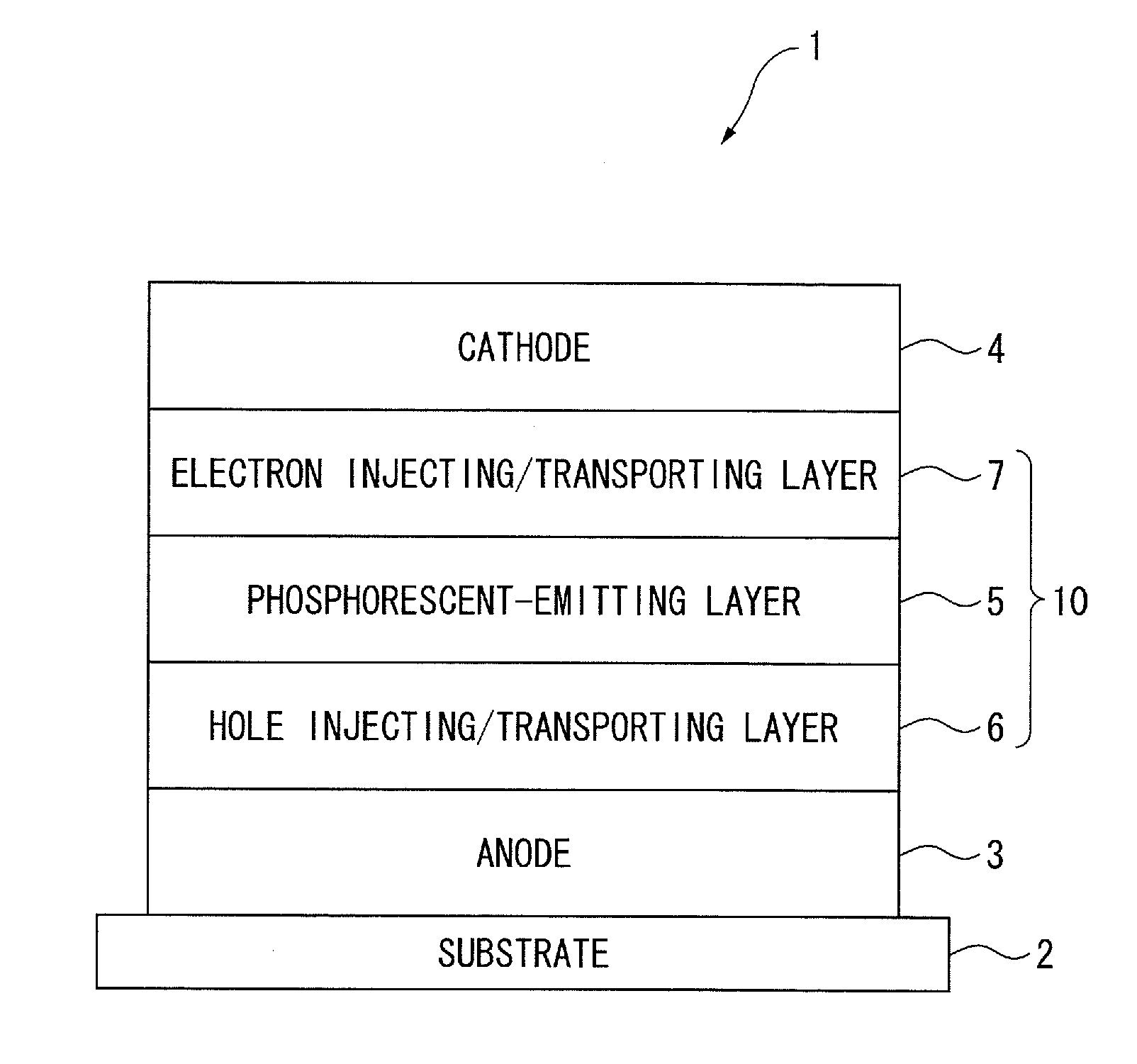
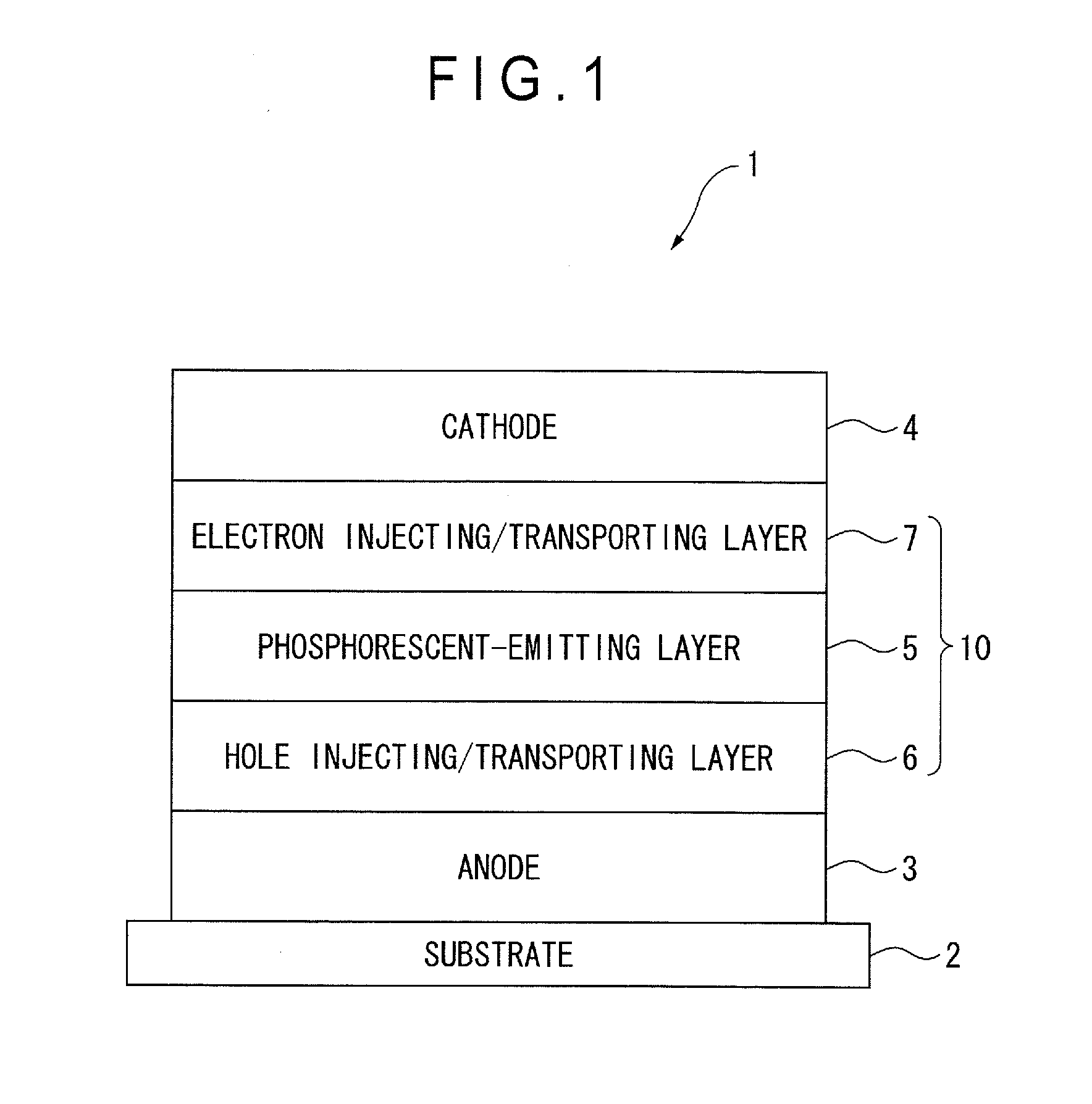
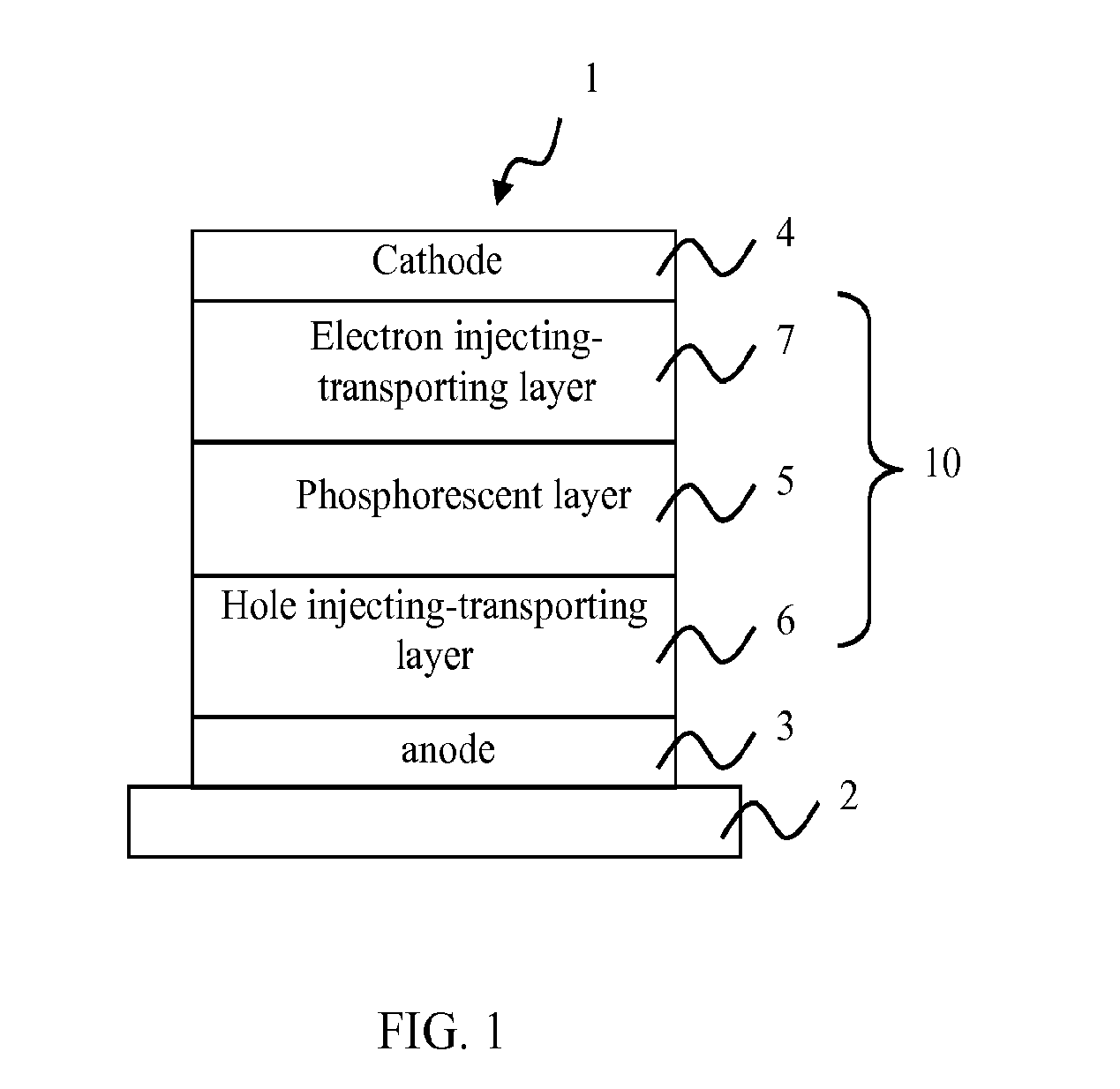
Material for organic electroluminescence device, and organic electroluminescence device using the same
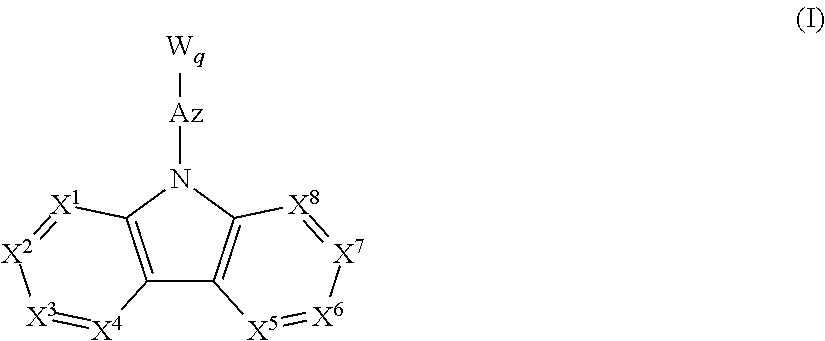
A material for an organic electroluminescence device represented by the following formula (I):wherein X1 to X8 are a nitrogen atom, CH, CHal or CRa; Az is a nitrogen-containing six-membered ring or a fused polycyclic group including a nitrogen-containing six-membered ring; W is an aromatic hydrocarbon group having 6 to 30 ring carbon atoms which is substituted by at least one cyano group or a heterocyclic group having 5 to 30 ring atoms which is substituted by at least one cyano group.
Owner:IDEMITSU KOSAN CO LTD
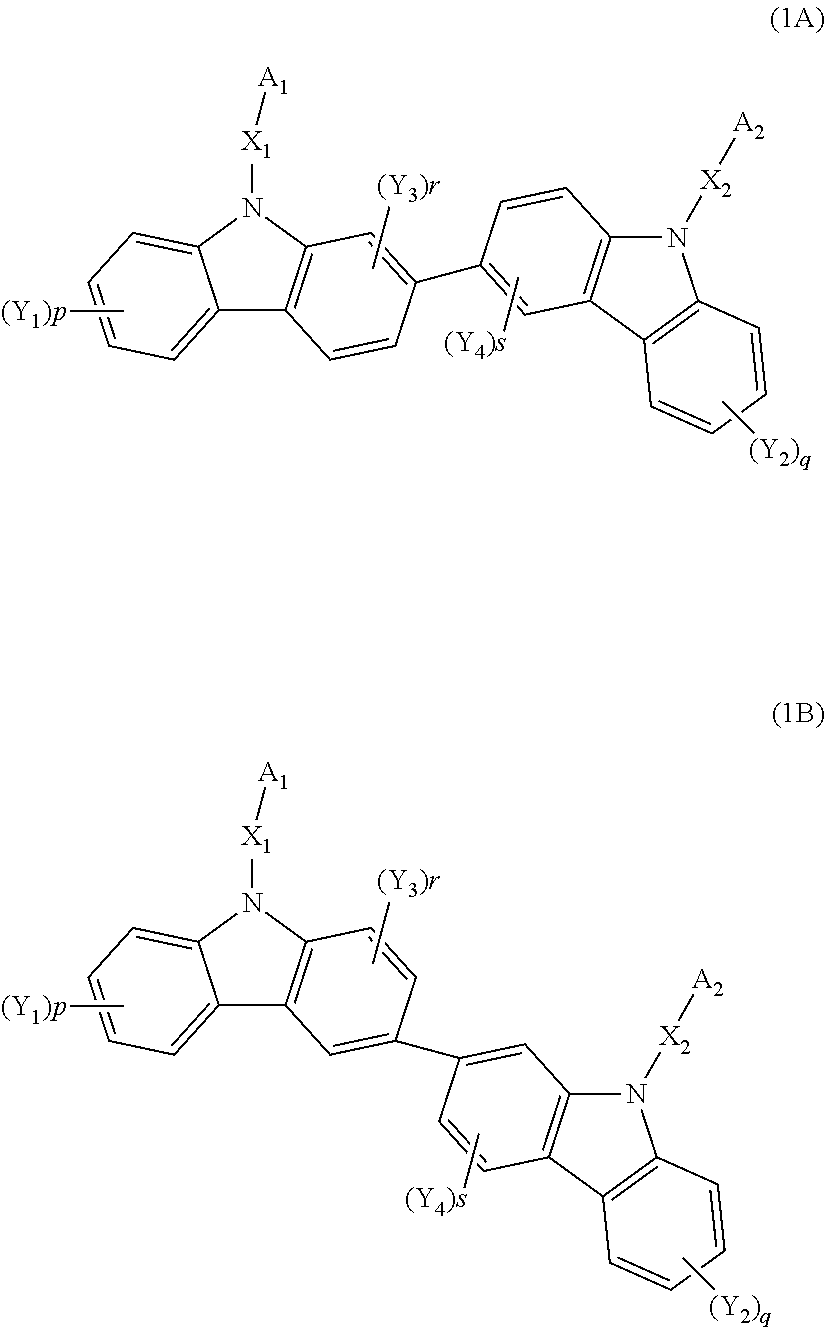
Biscarbazole derivative, material for organic electroluminescence device and organic electroluminescence device using the same
ActiveUS20120138912A1Improve balanceOrganic chemistryElectroluminescent light sourcesAnnulationOrganic electroluminescence
A biscarbazole derivative of the invention is represented by a formula (1) below.In the formula (1): A1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms; A2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms; X1 and X2 each are a linking group; Y1 to Y4 each represent a substituent; p and q represent an integer of 1 to 4; and r and s represent an integer of 1 to 3.
Owner:IDEMITSU KOSAN CO LTD
Synthetic method of plant growth regulator trinexapac-ethyl intermediate 3-carbethoxy-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate
ActiveCN102911058ANo purification requiredShort reaction timeOrganic compound preparationCarboxylic acid esters preparationKetoneSodium salt
The invention provides a synthetic method of plant growth regulator trinexapac-ethyl intermediate 3-carbethoxy-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate. The synthetic method comprises the following steps: (1) carrying out annulation reaction on 2-acetonyl-1,4-diethyl succinate and organic alkaline at the temperature of 20-120 DEG C for 0.5-5 hours in a non-polarity organic solvent to obtain 3,5-cyclohexanedione-1-ethyl formate; and (2) adding organic amine and cyclopropanecarboxylic acid chloride, and carrying out acylation reaction at the temperature of minus 5-50 DEG C, so as to obtain 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate in the presence of micromolecular alcohol, ether, ketone and nitrile the carbon atoms of which are below C8 and are used as additives. According to the method, special additives are added before acylation so that cyclopropanecarboxylic acid chloride can directly react with intermediate-state 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol sodium salt to obtain the trinexapac-ethyl precursor 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate, thereby shortening reaction time, simplifying synthesis process and improving yield; and the product is directly rearranged without purification so as to obtain the final product trinexapac-ethyl.
Owner:JIANGSU YOUJIA CHEM +1
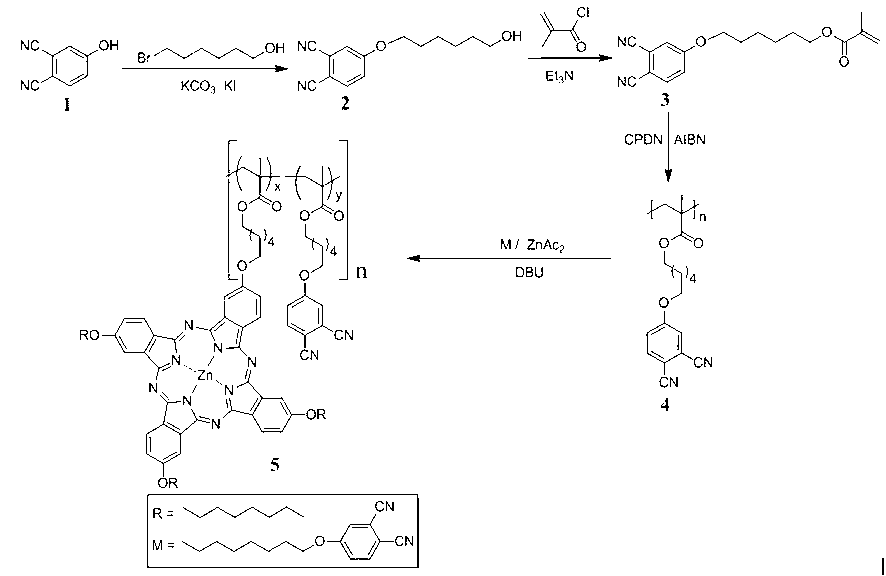
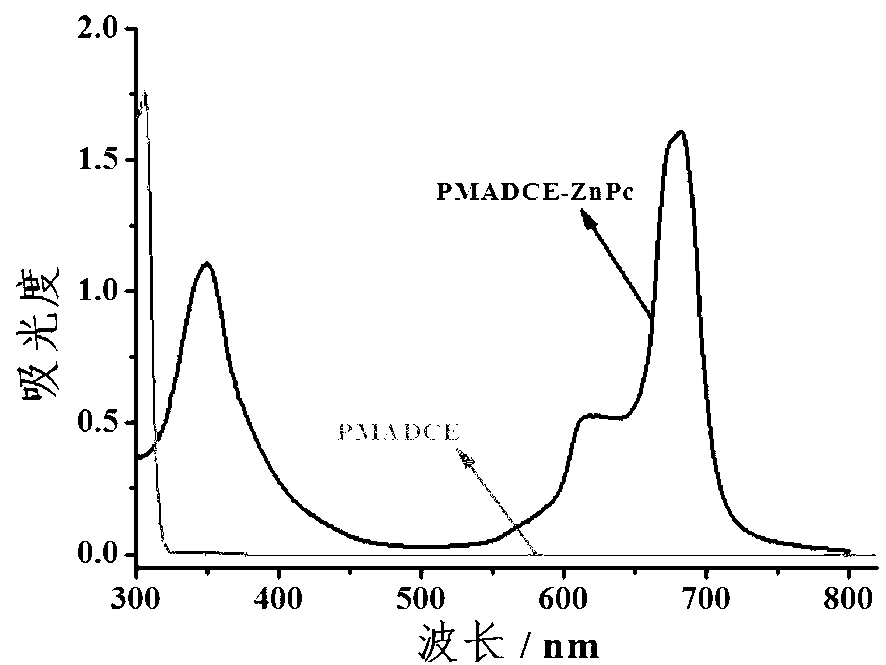
Copolymer containing zinc-phthalocyanine group on side chain and preparation method thereof
ActiveCN103193912AReduce wasteRealize rational utilizationSolid-state devicesSemiconductor/solid-state device manufacturingSolubilitySide chain
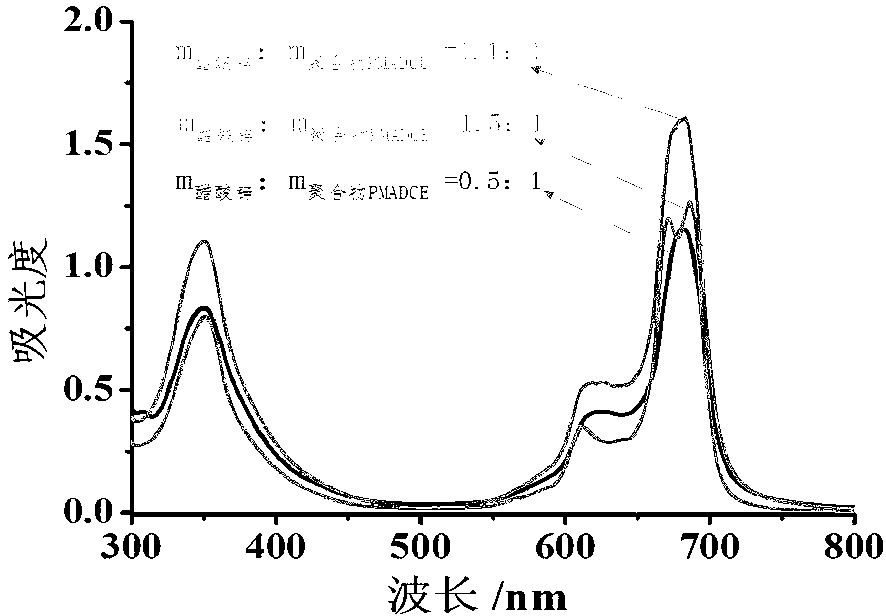
The invention discloses a copolymer containing a zinc-phthalocyanine group on a side chain and a preparation method thereof. The preparation method of the copolymer combines RAFT (reversible addition-fragmentation chain transfer) and a phthalocyanin annulation method for the first time to prepare the copolymer containing a zinc-phthalocyanine group on a side chain, and comprises the following steps of: on the presence of an initiator and a chain transfer agent, performing RAFT polymerization on a monomer methacrylic acid-6-(3,4-dicyanophenoxy)caprolactone to obtain a polymer PMADCE (polymethyl acrylic acid-6-(3,4-dicyanophenoxy)caprolactone) containing a phthalonitrile functional group on a side chain; and using the phthalonitrile functional group on the side chain of the PMADCE as a reaction point to perform phthalocyanin annulation with 4-(octyloxy)phthalonitrile in the presence of a heat-sensitive catalyst and zinc acetate so as to obtain a polymer PMADCE-ZnPc containing the zinc-phthalocyanine group on the side chain. The preparation method is few in step, simple, feasible, convenient in purification process, mild in reaction condition, controllable in process and high in conversion rate; and the PMADCE-ZnPc prepared by the method has good solubility and phthalocyanin functionality, can be used as a photoelectric material, and has good application prospects in solar cells, sensors and information storage.
Owner:蚌埠格识知识产权运营有限公司
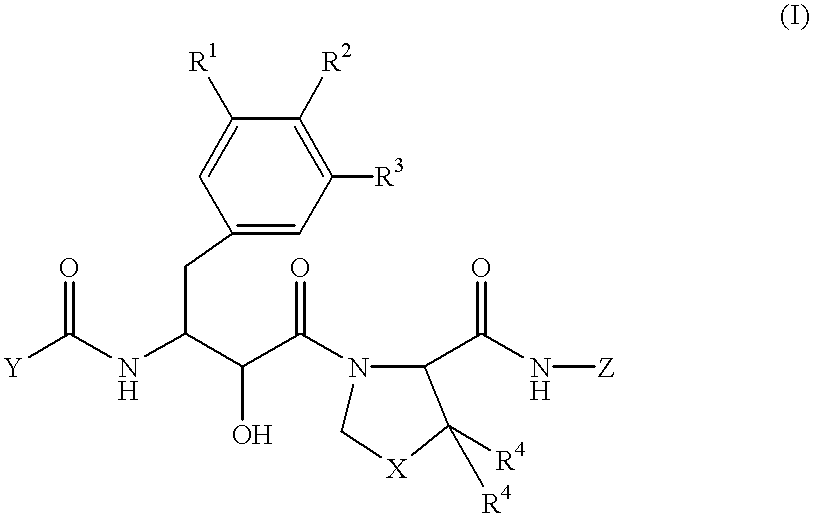
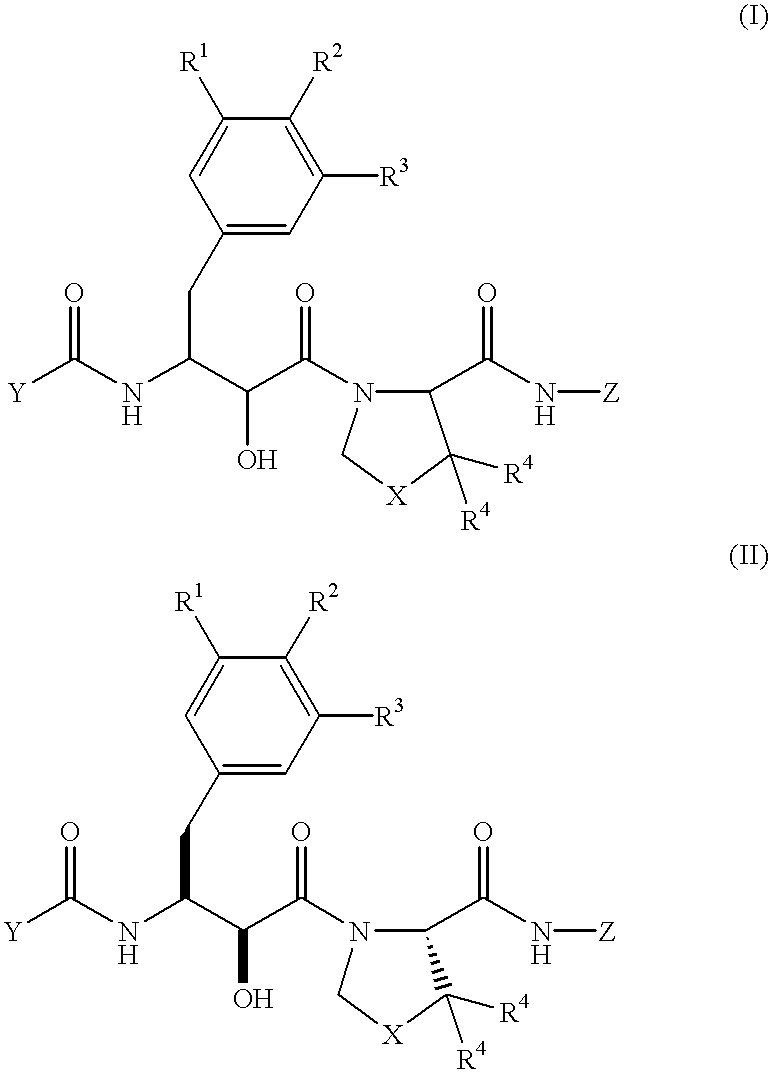
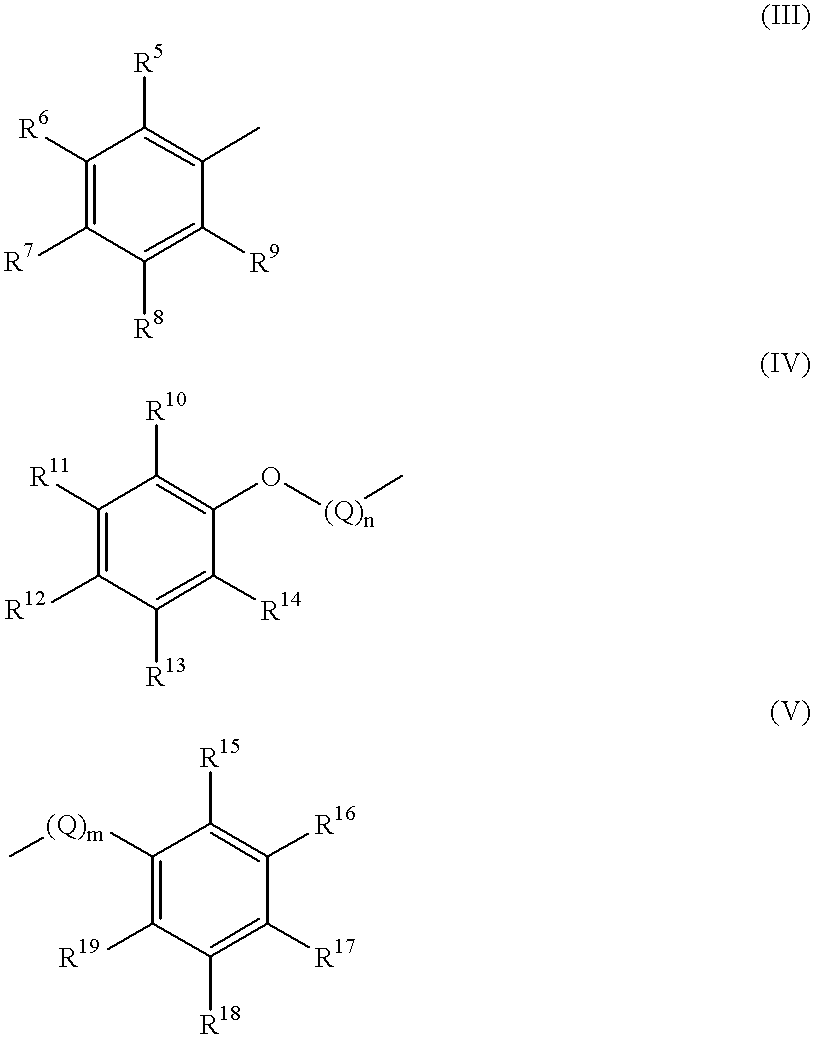
Novel dipeptide compounds and their use as medicines
A novel dipeptide compound inhibiting the enzymatic activity of HIV protease, an anti-AIDS medicine comprising this dipeptide compound as an effective component. The dipeptide compound is the compound represented by the formula (I) or a pharmaceutically acceptable salt thereof wherein R1, R2, and R3 independently represent a linear or branched, saturated or unsaturated lower alkyl group, alkoxyl group, alkyl amino group, or dialkyl amino group having 1-4 carbon atoms (with a carbon atom being either replaced by an oxygen atom or not), a halogeno group, or a hydrogen atom, provided that all of the R1, R2, and R2 are not a hydrogen atom at the same time, R2 and R3 may form a ring together, R4 represents a linear or branched lower alkyl group having 1-4 carbon atoms or a hydrogen atom; X is a methylene group or a sulfur atom; Y represents a five or six member monocyclic or polycyclic hydrocarbon group, a heterocyclic group having a structure in which one or more carbon atom in the monocyclic or polycyclic hydrocarbon group is replaced by a hetero atom, an aryloxy group having 12 or less carbon atoms, in which the aromatic ring may be substituted with an alkyl group, alkoxy group, halogeno group, amino group or hydroxyl group; and Z represents an aliphatic hydrocarbon group having 1-6 carbon atoms or an aromatic hydrocarbon group having 12 or less carbon atoms in which the aromatic ring may be substituted with an alkyl group, alkoxy group, or halogeno group, or one or more carbon atom in the aromatic hydrocarbon group may be replaced by a hetero atom.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Acid Secretion Inhibitor
ActiveUS20090118335A1Inhibit gastric acid secretionGood treatment effectAntibacterial agentsBiocideArylSulfur
The present invention provides a compound having a superior acid secretion inhibitory action, an antiulcer activity and the like.A proton pump inhibitor containing a compound represented by the formula (I)wherein ring A is a saturated or unsaturated 5- or 6-membered ring group optionally having, as a ring-constituting atom besides carbon atom, 1 to 4 hetero atoms selected from a nitrogen atom, an oxygen atom and a sulfur atom, ring-constituting atoms X1 and X2 are each a carbon atom or a nitrogen atom, a ring-constituting atom X3 is a carbon atom, a nitrogen atom, an oxygen atom or a sulfur atom, R1 is an optionally substituted aryl group or an optionally substituted heteroaryl group, R2 is an optionally substituted alkyl group, an optionally substituted aryl group or an optionally substituted heteroaryl group, R3 is an aminomethyl group optionally substituted by 1 or 2 lower alkyl groups, which is a substituent on a ring-constituting atom other than X1, X2 and X3, and ring A optionally further has substituent(s) selected from a lower alkyl group, a halogen atom, a cyano group and an oxo group, or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Process for producing high purity 1,4-dioxane
InactiveCN1473823AReduce manufacturing costTo satisfy the market's needsOrganic chemistryWastewaterAnnulation
The present invention discloses the production process of high purity 1, 4-dioxane. Diglycol material is produced into high purity 1, 4-dioxane via dewatering and annulation at 150-200 deg.c in the presence of liquid and solid catalysts; low temperature dewatering via adding azeotrope, and eliminating impurity with added impurity eliminating agent. The present invention has less catalyst consumption, high yield, low cost, and no waste water and waste gas exhausted.
Owner:徐汝华 +1
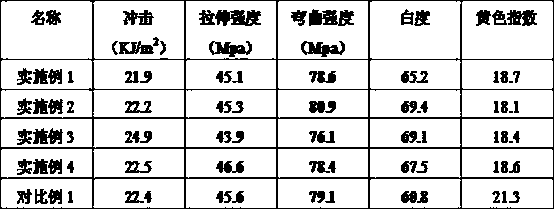
Preparation method of yellowing-resistant and high-brightness ABS resin
The invention relates to a preparation method of yellowing-resistant and high-brightness ABS resin and belongs to the field of synthetic resin. A functional monomer polymerized phosphate monomer is introduced in the synthetic process of ABS grafting powder and is chelated with metal ions in a matrix resin, so that the defects of the ABS resin are reduced, the thermo-oxidative aging resistance of the ABS resin is improved, the crosslinking aging level of butadiene double bonds in the resin is decreased, the annulation reaction among cyano groups is prevented, the yellow color index is decreased, and the whiteness is increased.
Owner:中化国际聚合物(连云港)有限公司
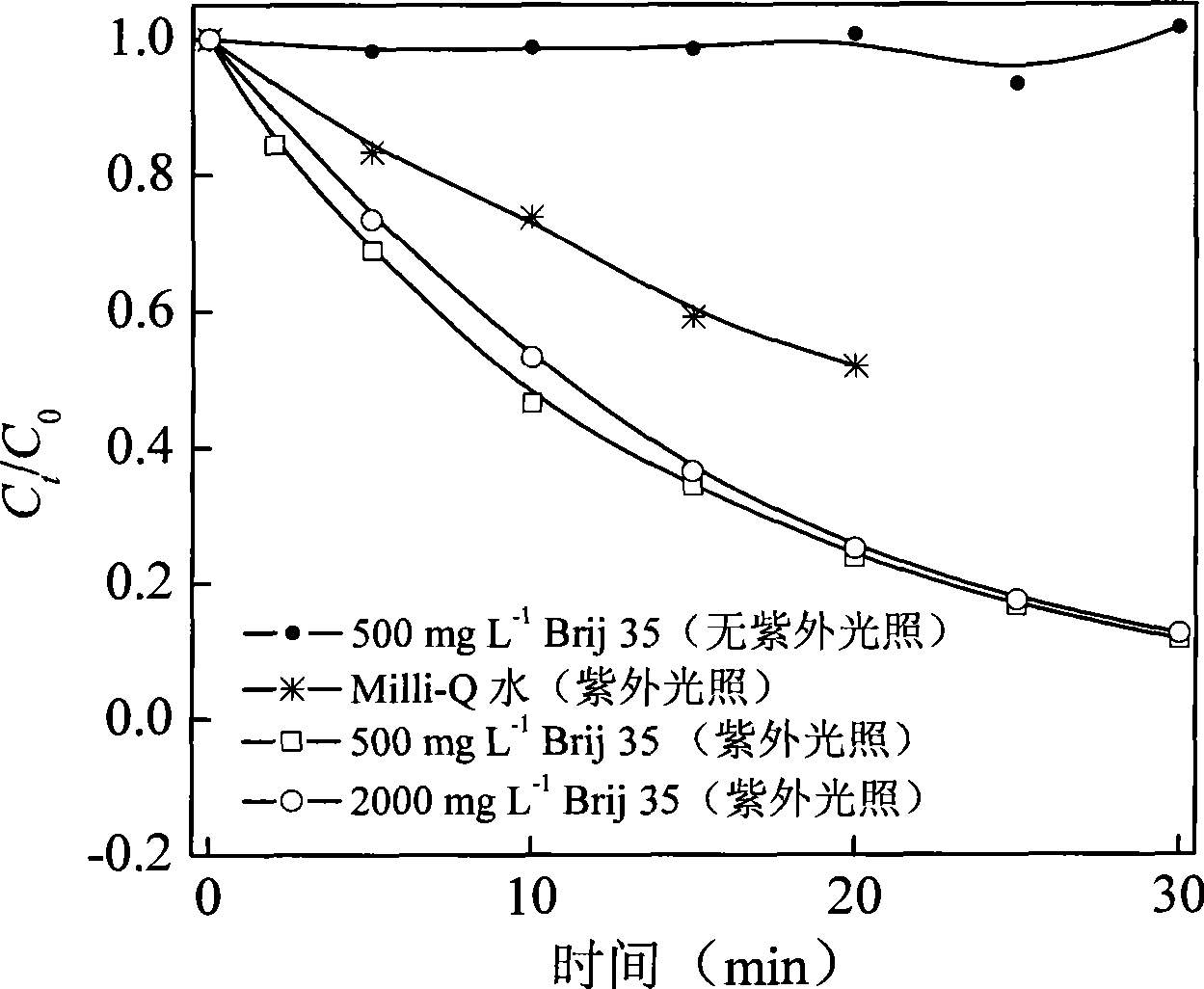
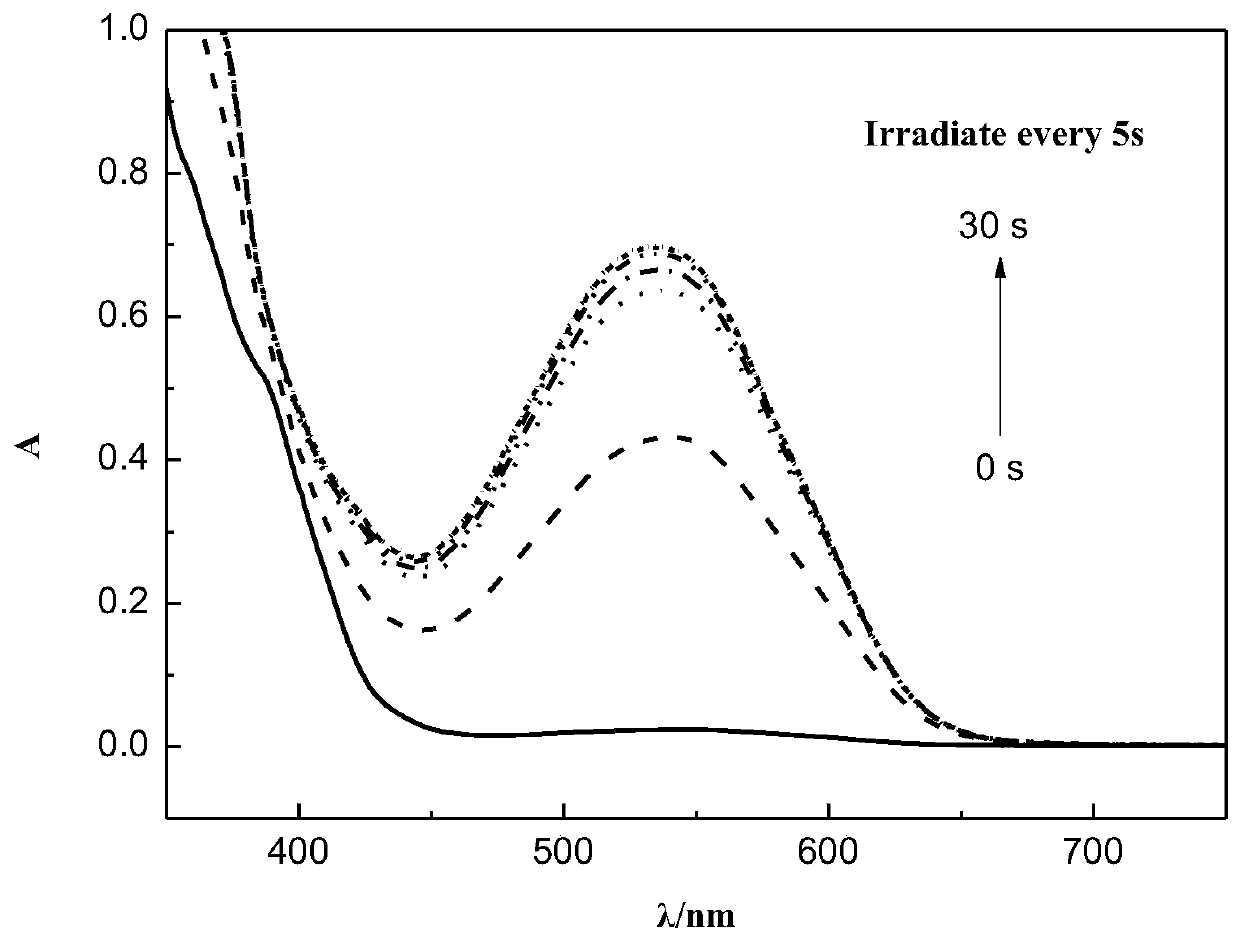
Method for degrading polybrominated diphenyl ethers using surface active agent solubilization combined with UV technique
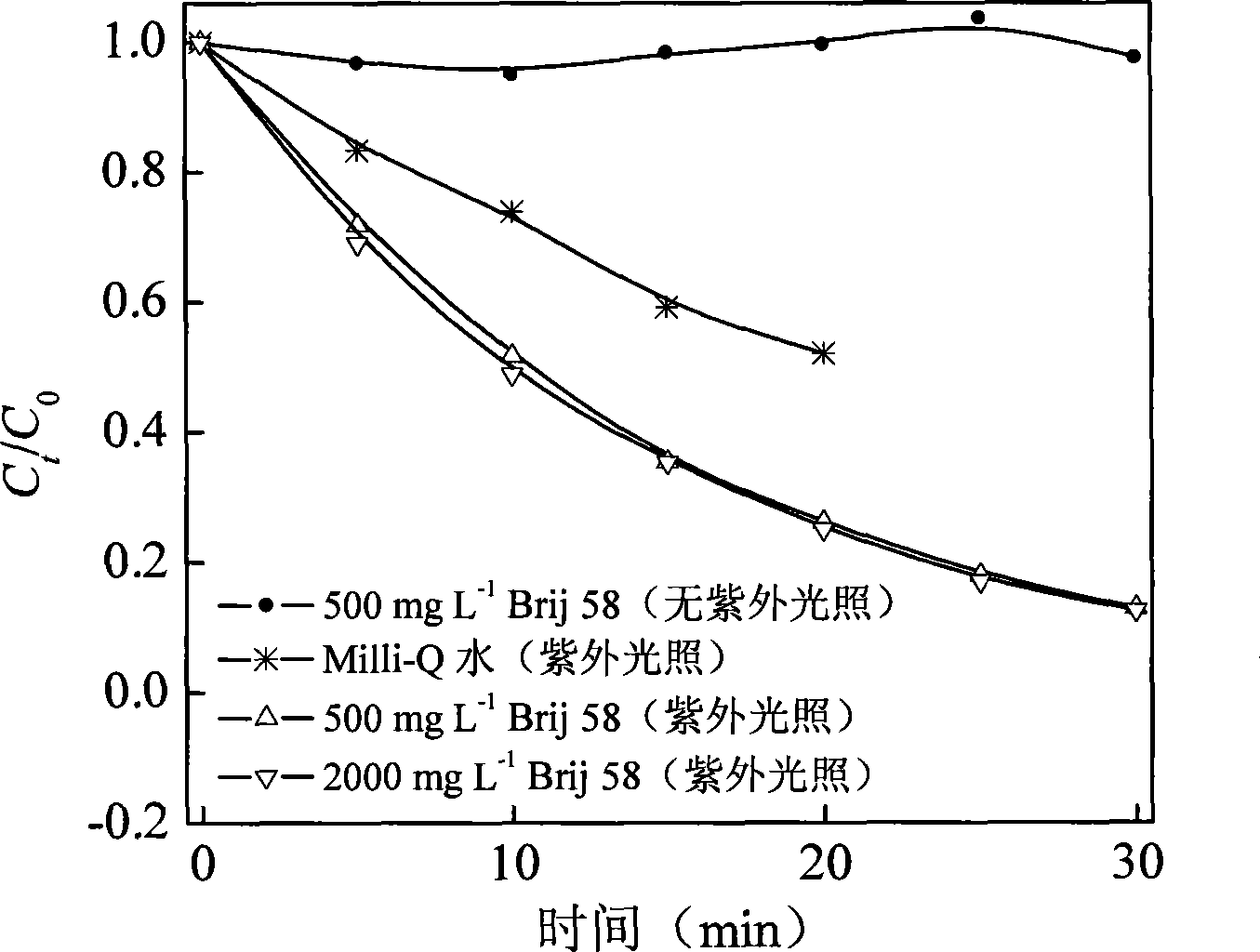
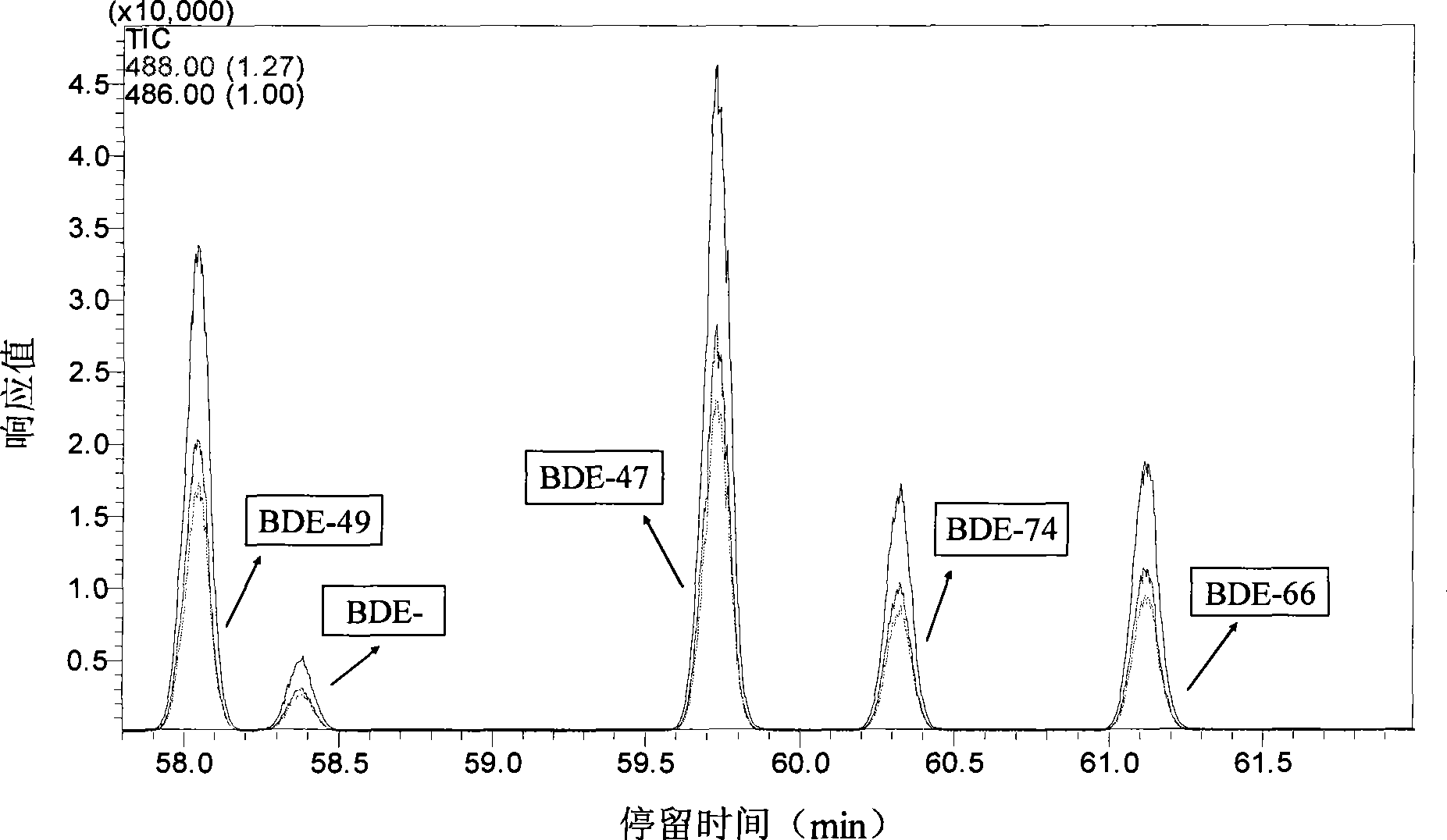
InactiveCN101461989AShort reaction timeImprove efficiencyChemical protectionWater bathsSelective degradation
The invention discloses a method for degrading polybrominated diphenyl ether by combining surfactant solubilization and UV technology, which belongs to the technical field of environment-friendly halogenated aryl hydrocarbon organic pollutant treatment. The method adopts the technical proposal that surfactant solubilization of 2,2',4,4',5-pentabromated diphenyl ether is improved in a hydrophobic environment provided by nonionic surfactant micelle (Brij 35 or Brij 38) under the conditions of normal temperature and pressure, water bath and ultrasonic sound; and the 2,2',4,4',5-pentabromated diphenyl ether which is dissolved in a surfactant solution is subjected to UV advanced treatment at normal temperature and pressure and then subjected to stepwise debromination reaction or intermolecular elimination and annulation reaction to generate intermediate products, namely PBDEs from monobromo to tetrabromo and polybrominated dibenzofuran (PBDFs) from monobromo to tetrabromo. The whole technology has short time consumption and high efficiency, and the method can selectively degrade target pollutant without damaging a surfactant, provides convenience for recycling of the surfactant, and has good application prospect in the aspect of remediation of soil polluted by the PBDEs in electronic waste recycling sites.
Owner:TSINGHUA UNIV
Benzoxazine resin based carbon aerogel and a preparing method thereof
Benzoxazine resin based carbon aerogel and a preparing method thereof are disclosed. The method includes weighing an amine and paraformaldehyde, adding into a four-neck distillation flask, adding an organic solvent, stirring at room temperature until full dissolution, weighing a phenol monomer, dissolving the monomer into the organic solvent, mixing the obtained solution with the solution mentioned above, stirring, gradually heating to allow the amine, the paraformaldehyde and the phenol monomer to be subjected to an annulation reaction to obtain a benzoxazine solution, heating the benzoxazine solution in an oil bath to cure the benzoxazine solution so as to prepare partially-cured polybenzoxazine wet gel, naturally drying the partially-cured polybenzoxazine wet gel to prepare partially-cured polybenzoxazine aerogel, and subjecting the cured polybenzoxazine aerogel to heat treatment and high-temperature carbonization in an inert atmosphere to prepare polybenzoxazine resin based carbon aerogel. The product has advantages of a short preparation period, a low production cost, low equipment requirements, high safety, and high product purity.
Owner:TAISHAN MEDICAL UNIV
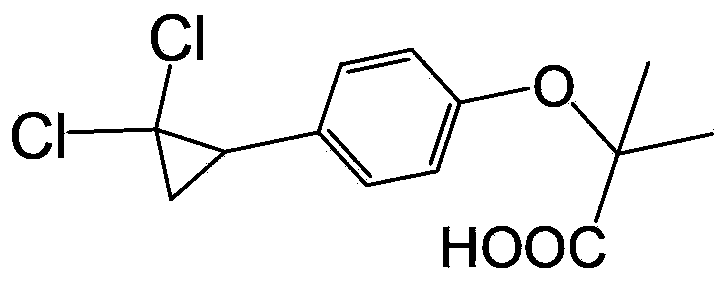
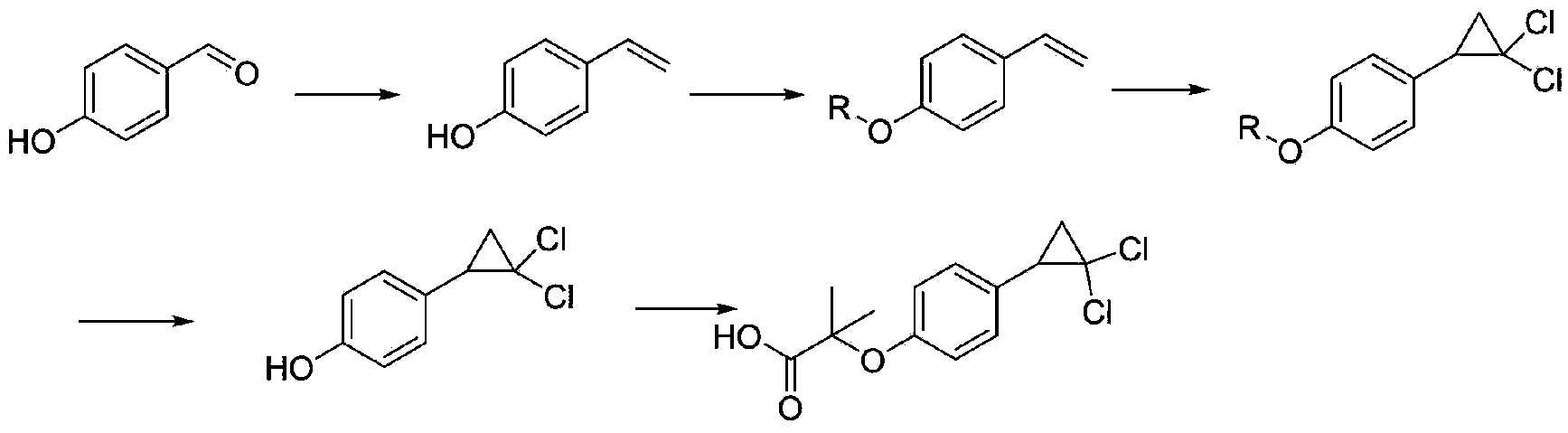
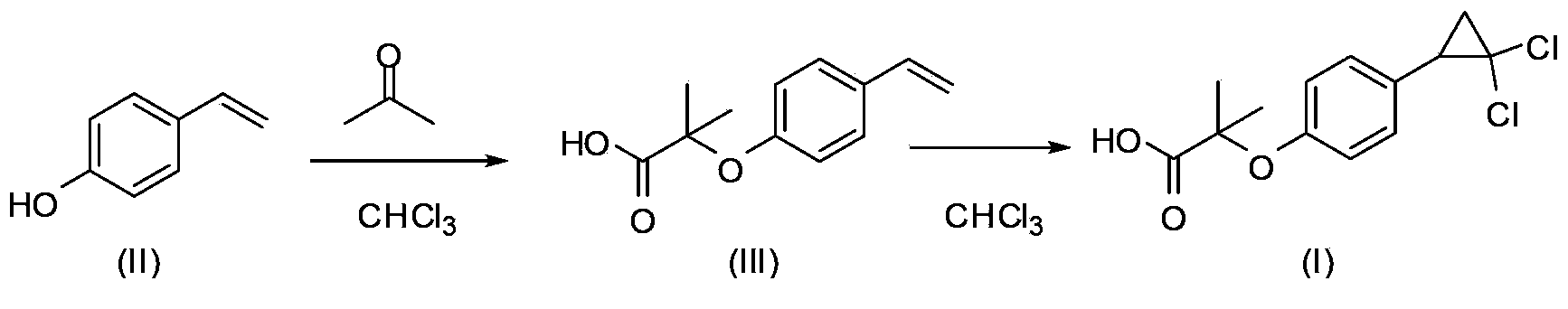
Environment-friendly preparation method for lipid-lowering drug ciprofibrate
ActiveCN103373916AImprove friendlinessNo emissionsOrganic compound preparationCarboxylic compound preparationAnnulationReaction step
The invention provides an environment-friendly preparation method for a lipid-lowering drug ciprofibrate. The preparation method comprises the following steps of by taking hydroxyphenyl ethene as a raw material, carrying out Bargellini and annulation reaction to prepare the ciprofibrate. Compared with the traditional method, the method has the advantages of less reaction steps, short production cycle and high total yield, is easy to operate, mild and easy-control in conditions, convenient for postprocessing and environment-friendly, and is a brand new method for industrialized synthesis of the ciprofibrate.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Substituted arylalkanoic acid derivatives and use thereof
InactiveUS20070213333A1Superior prophylactic and therapeutic effectLow toxicityBiocideOrganic compound preparationCarbon atomAryl
A compound represented by the formula (I): [In the formula, Link represents a saturated or unsaturated straight hydrocarbon chain having 1 to 3 carbon atoms, C2 to C6 in the aromatic ring (E) independently represent a ring-constituting carbon atom, one of the ring-constituting carbon atoms may be replaced with V, V represents nitrogen atom, or carbon atom substituted with Zx, Zx represents a saturated alkyl group having 1 to 4 carbon atoms and the like, Rs represents -D-Rx etc., D represents a single bond, oxygen atom and the like, Rx represents a saturated alkyl group having 3 to 8 carbon atoms and the like, AR represents a partially unsaturated or completely unsaturated condensed bicyclic carbon ring or a heterocyclic ring, and Y represents hydrogen atom, a lower alkyl group having 1 to 4 carbon atoms and the like] or a salt thereof. A compound having prostaglandin production-suppressing action and leukotriene production-suppressing action is provided.
Owner:ASAHI KASEI PHARMA
Annulation and matched handset wore on wrist
InactiveCN101094054ANot easy to loseEasy to useSecuring communicationTelephone set constructionsAnnulationEngineering
Via its info storing and processing functions (ISP), one side or both sides of the annulation and its matched handset (MH) can be used to keep the secret key or other important info. MH may become the complementary or unique device to control the annulation and the on / off of its ISP. When the annulation and its ISP are on, ISP of MH can control the working state of the info managing system of MH depending on the distance of wireless connection with MH. Wearing on the wrist, this annulations and its MH are not easy to drop but easy to use.
Owner:盛年
Curing compositions for fluoropolymers
ActiveCN101080448AGood physical propertiesSmall compression setThin material handlingArylPolymer science
A curative composition comprising a cation and an anion of the formula (I): wherein m, n, p, and q are positive integers, wherein m * p = n * q, wherein Q is an organo onium, and A is an anion, provided that at least one A is selected from the formula (II): wherein each R independently is H, halo, alkyl, aryl, aralkyl, or cycloalkyl, and which also may be halogenated, fluorinated, or perfluorinated, wherein two or more of R and R' groups may together form a ring, wherein each R group independently may contain one or more heteroatom(s), wherein R' can be the same as R, with the proviso that R' cannot be halo. Also provided are a fluoropolymer composition including this curative, a method of making a fluoropolymer, and fluoropolymer articles containing curable or cured fluoropolymer compositions.
Owner:3M INNOVATIVE PROPERTIES CO
Organic electroluminescence material and application thereof
InactiveCN110759851AIncrease RISC speedEffective use of triplet energy levelsOrganic chemistrySolid-state devicesArylSimple Organic Compounds
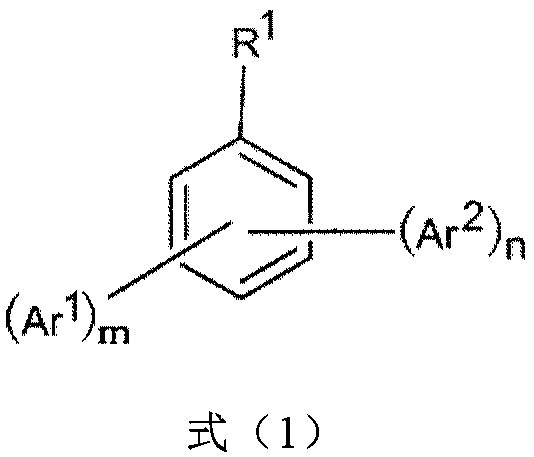
The invention discloses an organic compound which has a structural formula as shown in a formula (1). In the formula (1), R1 is selected from CN, CF3 and NO2; m is an integer from 0 to 2, and n is aninteger from 2 to (5-m); Ar1 is selected from hydrogen, halogen, C1-C8 alkyl, C5-C10 cycloalkyl, C1-C8 alkoxy, C6-C14 aryl and C3-C12 heteroaryl, and multiple Ar1s can be the same or different; Ar2 has a structural formula as shown in a formula (2), and multiple Ar2s can be the same or different; in the formula (2), X is selected from single bonds, O, S, NR8 and CR9R10; * denotes the position of connection between the Ar2 and a benzene ring in the formula (1); each of R2-R7 is independently selected from hydrogen or C1-C14 electron donating group, and at least one is C3-C12 non-tertiary-carbonbranched alkyl; any two of the R2-R7 can form a ring; and each of R8-R10 is independently selected from hydrogen, halogen, carbonyl, C1-C8 alkyl, C5-C10 cycloalkyl, C1-C8 alkoxy, C6-C14 aryl and C3-C12 heteroaryl or a combination thereof.
Owner:BEIJING ETERNAL MATERIAL TECH
Tricyclic compound, process for producing the same, and use
A compound of the formula: wherein R1 is a 5- or 6-membered ring; Z1 is a 5- or 6-membered aromatic ring; Z2 is a group -Z2a-W2-Z2b-, wherein Z2a and Z2b are each O, S(O)q (wherein q is 0, 1 or 2), an imino group, or a bond; and W2 is an alkylene chain; W is a group represented by wherein R3 and R3′ are each a hydrogen atom, a lower alkyl group, or a lower alkoxy group; X is CH or N; n and n′ are each an integer of 0 or 1 to 4; m and m′ are each 1 or 2; Y is O, S(O)p (wherein p is 0, 1 or 2), CH2 or NR4 (wherein R4 is a hydrogen atom, a lower alkyl group, or a lower acyl group); and R2 is (1) an amino group, in which the nitrogen atom may be converted to a quaternary ammonium or an oxide, or (2) a nitrogen-containing heterocyclic group which may contain a sulfur atom or an oxygen atom as the ring-constituting atom, in which the nitrogen atom may be converted to a quaternary ammonium or an oxide; or a salt thereof. The compound exhibits excellent CCR antagonist activity against CCR5, and is useful as a prophylactic and / or therapeutic agent for HIV infection in human peripheral blood mononuclear cells, especially for AIDS.
Owner:SHIRAISHI MITSURU +4
Enantioselective N-heterocyclic carbene-catalyzed annulation reactions with imidazolidinones
Enantiomeric bicyclic lactone compounds as can be prepared via an N-heterocyclic carbene-catalyzed annulation reaction.
Owner:NORTHWESTERN UNIV
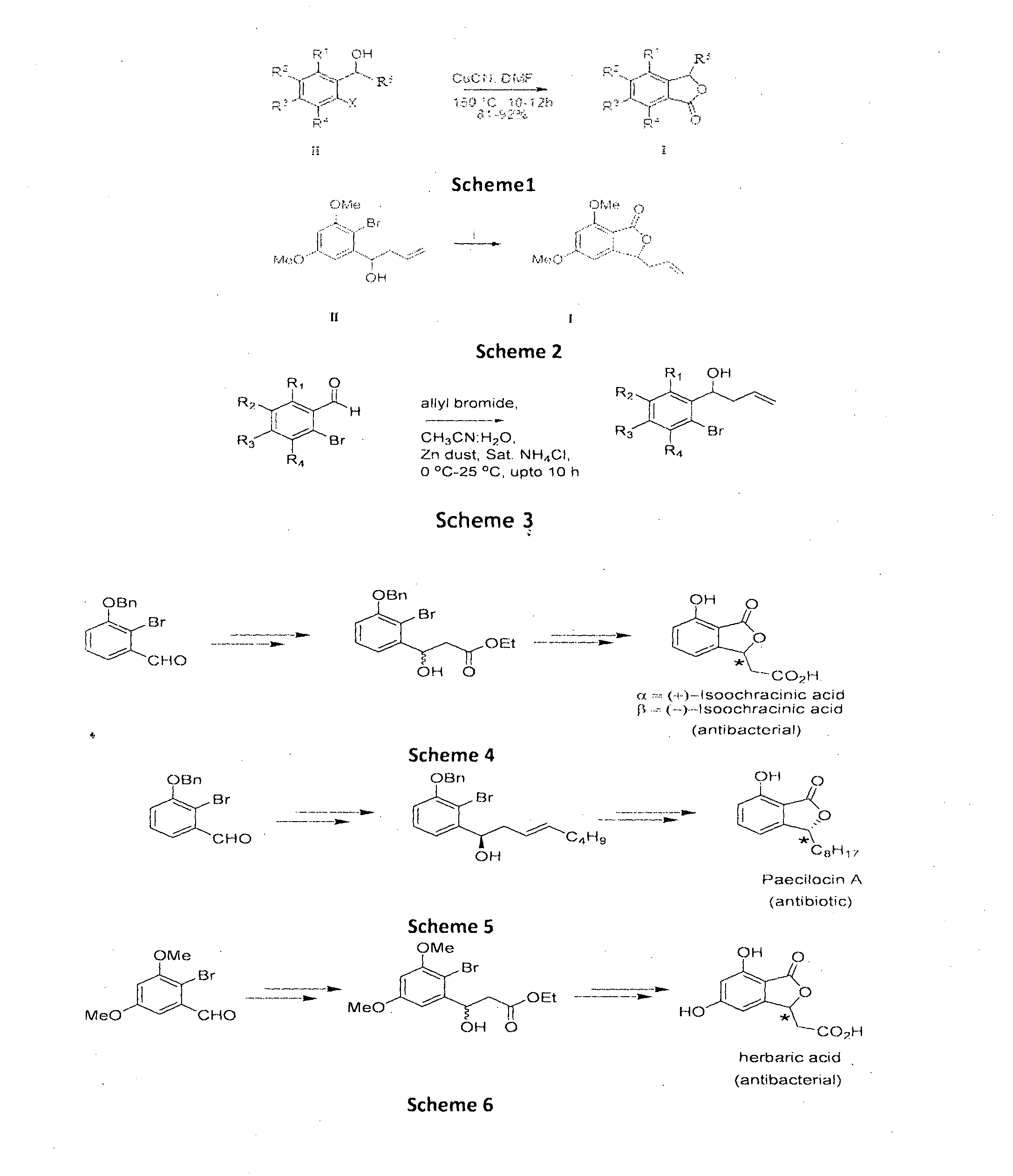
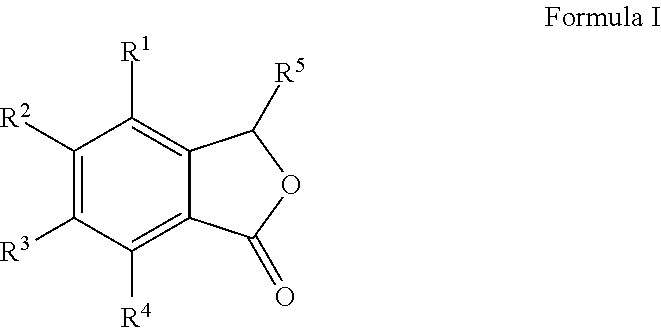
Cu-MEDIATED ANNULATION FOR THE EFFECTIVE SYNTHESIS OF 3-SUBSTITUTED PHTHALIDES
The present invention disclosed herein is a novel commercially feasible, one pot synthesis of library of 3-substituted phthalides of formula I via CuCN mediated oxidative cyclization in high yield. Formula I
Owner:COUNCIL OF SCI & IND RES
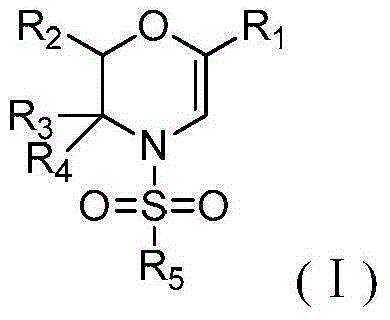
Method for preparing N-sulfonyl-1,4-oxazine derivative
The invention discloses a method for preparing a N-sulfonyl-1,4-oxazine derivative, in an organic solvent, tetracarboxylic rhodium is taken as a catalyst, a 1-sulfonyl-1,2,3-triazole compound and an epoxy compound are taken as substrates, so that the N-sulfonyl-1,4-oxazine derivative can be obtained. According to the invention, the N-sulfonyl-1,2,3-triazole compound and the epoxy compound are taken as the substrates for nitrogen removal and annulations reactions, so that N-sulfonyl-1,4-oxazine derivative can be obtained. The reaction raw materials have the advantages of simple preparation method, easy preservation, little rhodium catalyst usage, and greatly cost reduction. The method can be used for synthesizing a series of the N-sulfonyl-1,4-oxazine derivative, and the synthesized products have biological activity.
Owner:ZHEJIANG UNIV
Preparation method of allyl chlorooxyl cephalosporin compound
The invention relates to a preparation method of an allyl chlorooxyl cephalosporin compound, and belongs to the technical field of pharmaceutical synthesis. In order to solve the technical problem that an existing process is long in line and low in product yield and purity, the invention provides the preparation method of the allyl chlorooxyl cephalosporin compound. The method comprises the following steps of: performing an additive reaction on an allyl compound and chlorine to obtain a reaction liquid of a dichloro product; then, adding organic alkali for elimination reaction to obtain a compound shown as a formula III; then, performing a hydrolysis reaction of the compound shown as the formula III in a mixed solvent including dimethyl sulfoxide and water under the catalytic effect of an acidic catalyst and Cu2O to obtain an allyl alcohol intermediate; then, performing annulation under the effect of boron trifluoride diethyl etherate or methanesulfonic acid to obtain a compound shown as a formula V; and then, under the effect of an initiator, performing chlorination of the compound shown as the formula V and a chlorinating agent to obtain the allyl chlorooxyl cephalosporin compound. The method has the advantages of simple process and high product purity and yield.
Owner:浙江东邦药业有限公司
N-sulfinyl amino acid amide compound and application thereof
InactiveCN101597247AHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAmino acidImine
The invention discloses an optical pure N-sulfinyl amino acid amide compound and an application thereof, belonging to the technical field of organic chemistry. The optical pure N-sulfinyl amino acid amide compound has a structural formula shown in the right. Configurations of a sulfur atom and Cl are respectively R or S, wherein R and R are respectively an alkyl containing 1-25 of carbon atoms or an aryl containing 6-50 of carbon atoms; R is hydrogen or the alkyl containing 1-25 of carbon atoms or the aryl containing 6-50 of carbon atoms; R is a common pendant amino acid group comprising an annular pendant amino acid group forming a ring with the Cl, N and the R. The compound can selectively catalyze the hydrogen transfer reduction reaction of imine by trichlorosilane in three dimensions.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Synthetic method of 2-chloro-4-phenyl quinazoline
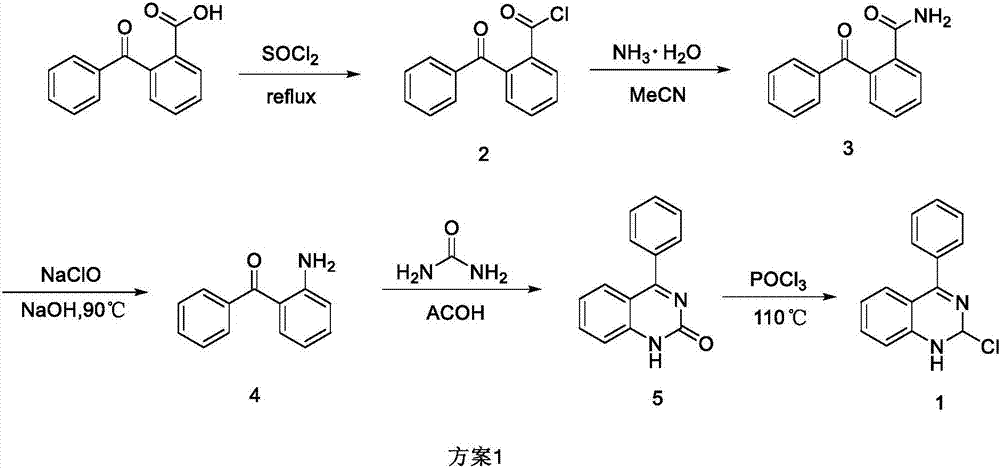
InactiveCN107082765ASimple post-processingRealize scale-up productionOrganic chemistryOrganic synthesisBenzoyl chloride
The invention relates to a synthetic method of 2-chloro-4-phenyl quinazoline and belongs to the technical field of organic synthetic chemistry. The synthetic method comprises the following steps of: by taking o-benzoylbenzoic acid as a raw material, performing an acylating chlorination reaction to generate o-benzoyl benzoyl chloride; performing an amidation reaction to generate o-benzoyl benzamide; performing a Hofmann degradation reaction to generate 2-amino benzophenone; and performing an annulations reaction to generate 4-phenyl quinazoline-2(1H)-one and chlorinating the 4-phenyl quinazoline-2(1H)-one by phosphorus oxychloride to finally generate 2-chloro-4-phenyl quinazoline, wherein the total yield is 20.8%. Compared with other methods, the reagents used by the route are low in price and easily available, and the post-treatment is simple. In the reaction process, the reaction conditions and synthetic processes thereof are explored, so that a simple and feasible synthetic route is provided for synthesizing 2-chloro-4-phenyl quinazoline, thereby realizing amplified production.
Owner:CHANGZHOU UNIV
Process for the preparation of furan compounds
InactiveUS7608727B2Efficient preparationEasily and conveniently produceOrganic chemistryFuranHalogen
A carbonyl compound represented by following Formula (1):wherein R1 represents hydrogen atom or an organic group; and R2 represents hydrogen atom or an organic group having a carbon atom at a bonding site with the carbonyl group in Formula (1), wherein R1 and R2 may be combined to form a ring with adjacent two carbon atoms, or an equivalent thereof is reacted with an unsaturated compound represented by following Formula (2):wherein each of R3, R4, and R5 represents hydrogen atom, a halogen atom, hydroxyl group, or an organic group and wherein R3 and R4 may be combined to form a ring with adjacent two carbon atoms, or a precursor thereof, to yield a furan compound represented by following Formula (3):wherein R3′ represents R3, R5 or hydrogen atom; and R1, R2, R3, R4, are R5 are as defined above.
Owner:DAICEL CHEM IND LTD
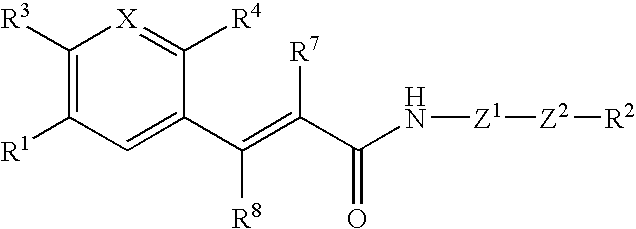
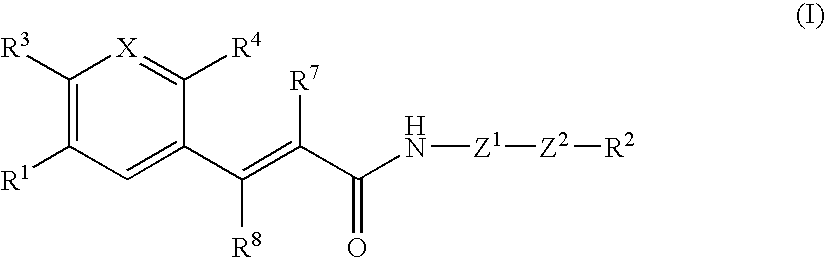
Acrylamide derivative, process for producing the same, and use
InactiveUS20060160864A1Enhance pharmacological effectsHigh activityBiocideOrganic chemistryChemical compoundAcyl group
A compound represented by the formula: wherein R1 is a 5- or 6-membered ring; R3 is a hydrogen atom, a lower alkyl group or a lower alkoxy group; R7 and R8 are each a hydrogen atom or a lower alkyl group; Z1 is another 5- or 6-membered aromatic ring; Z2 is a group represented by -Z2a-W1-Z2b- [wherein Z2a and Z2b are each O, S(O)m (wherein m is 0, 1 or 2), an imino group or a bond, and W1 is an alkylene chain]; X is CR (wherein R is a hydrogen atom, a lower alkyl group, a lower alkoxy group, an acyl group, or R and adjacent R4 may form a 5- or 6-membered alicyclic heterocyclic group) or N; R4 is NR5R6 (wherein R5 and R6 are each a hydrogen atom, a hydrocarbon group, a heterocyclic group or an acyl group), or R5 and R6 are bonded to each other to form a heterocyclic group of NR5R6; and R2 is (1) an amino group which may be a quaternary ammonium or oxide, (2) a nitrogen-containing heterocyclic group which may contain a sulfur atom or an oxygen atom as the ring-constituting atom, in which the nitrogen atom may be converted to a quaternary ammonium or an oxide, or the like; or a salt thereof. The compound has excellent CCR5 antagonistic activity and thus is useful as a prophylactic and / or therapeutic medicine for HIV infection into human peripheral blood monocyte, especially for AIDS.
Owner:TAKEDA PHARMA CO LTD
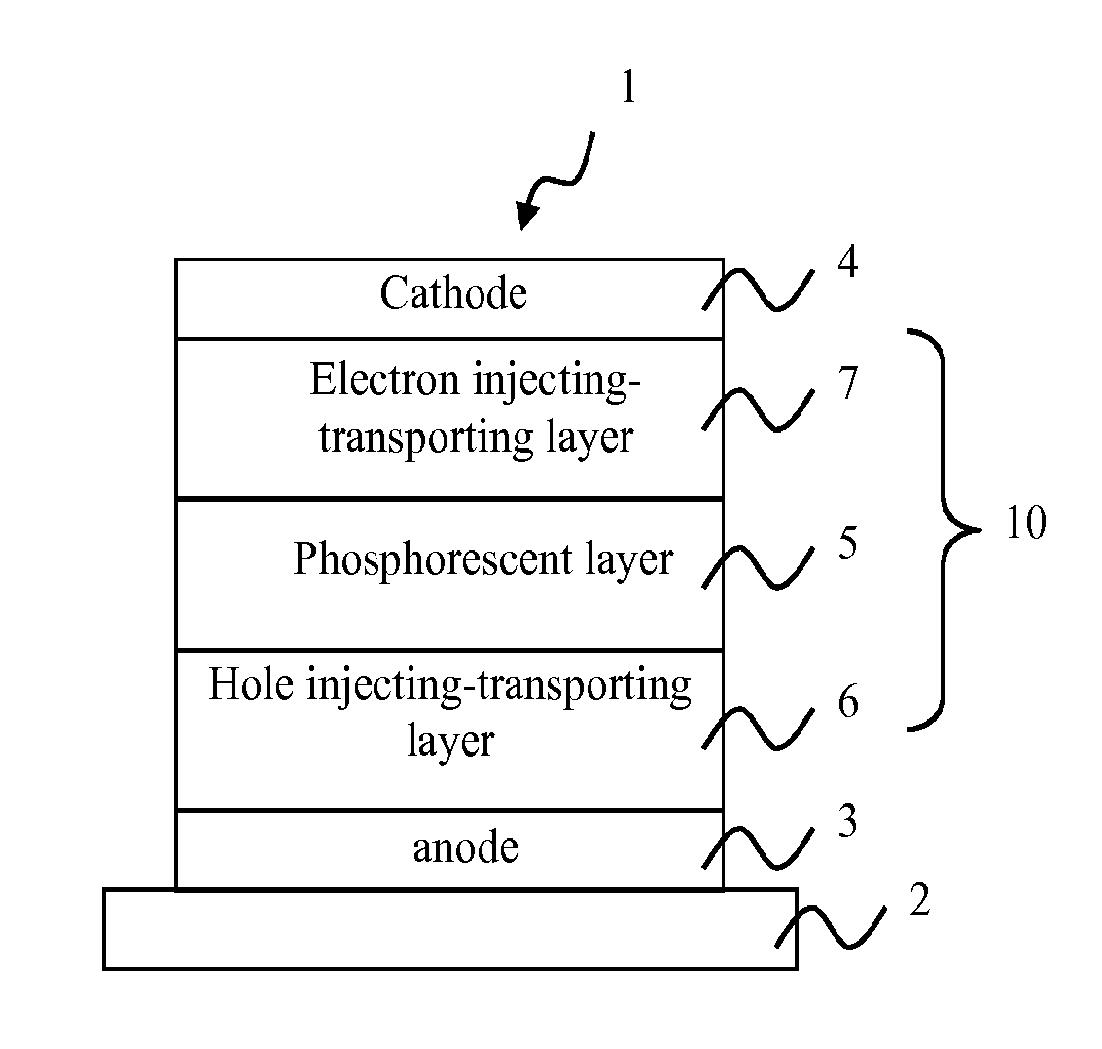
Biscarbazole derivative host materials and green emitter for OLED emissive region
ActiveUS20150243905A1Low voltage requirementHigh luninous efficiencyOrganic chemistrySolid-state devicesOrganic electroluminescenceCoordination complex
An organic electroluminescence device utilizes a novel combination comprising one or more biscarbazole derivative compounds as the phosphorescent host material in combination with a green phosphorescent dopant material in the light emitting region of the device, where the biscarbazole derivative compounds are represented by a formula (1A) or (1B) below; where A1 represents a substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms; A2 represents a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 ring carbon atoms, or substituted or unsubstituted nitrogen-containing heterocyclic group having 1 to 30 ring carbon atoms; X1 and X2 each are a linking group; Y1 to Y4 each represent a substituent; p and q represent an integer of 1 to 4; and r and s represent an integer of 1 to 3; and the green phosphorescent dopant material is a phosphorescent organometallic complex having a chemical structure represented by LL′L″M wherein M is a metal that forms octahedral complexes, L, L′, and L″ are equivalent or inequivalent bidentate ligands wherein each L comprises a substituted or unsubstituted phenylpyridine ligand coordinated to M through an sp2 hybridized carbon and N; and, one of L, L′ and L″ is different from at least one of the other two.
Owner:UNIVERSAL DISPLAY +1
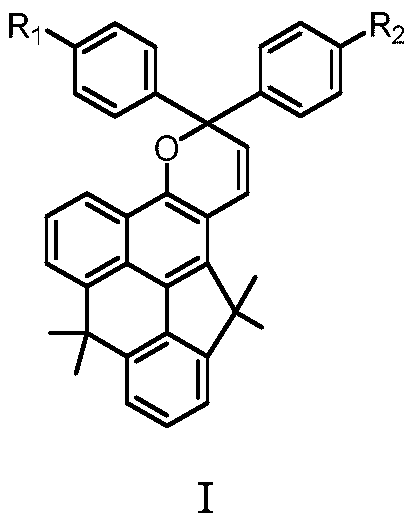
Double-condensed-ring naphthopyran photochromic compound and preparation method thereof
ActiveCN110343084AProcess reversibleQuick responseOrganic chemistryTenebresent compositionsPalladium catalystLight response
The invention relates to the field of organic functional materials. The invention provides a double-condensed-ring naphthopyran photochromic compound shown as a formula I and a preparation method thereof. The preparation method comprises the following steps: 4-methoxynaphthalen-1-ylboronic acid pinacol ester reacts with 2-bromoisophthalic acid dimethyl ester under the condition of a palladium catalyst to obtain 2-(4-methoxynaphthalen-1-yl)isophthalic acid dimethyl ester M1; the M1 and methylmagnesium bromide are subjected to addition, acidification cyclization and demethylation reactions to obtain 1,1,5,5-tetramethyl-1,5-dihydrobenzo[mno]aceanthrylene-9-ol M4; and then the compound M4 and 1,1-diaryl-2-propyn-1-ol react to generate the target compound I under acid catalysis. The compound Ican be changed from colorless to purple or blue under ultraviolet irradiation, the process is reversible, and the compound I has the characteristics of quick light response, excellent fatigue resistance, high color rate and the like. In the formula I, R<1> and R<2> are the same or different and separately represent hydrogen, straight-chain or branched-chain alkyl containing 1-6 carbon atoms, straight-chain or branched-chain alkoxy containing 1-6 carbon atoms, aryl, halogens, NH2 and -NRR.
Owner:TIANJIN UVOS TECH CO LTD +1
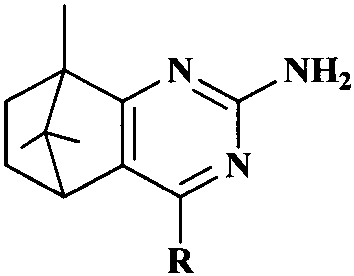
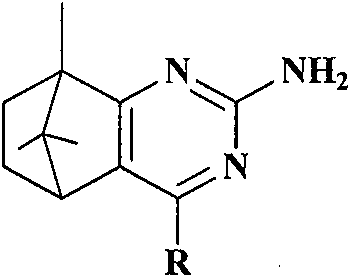
Synthesis and anti-tumor activity of camphoryl pyrimidines
InactiveCN110551070ARich sourcesEase of industrial productionOrganic chemistryAntineoplastic agentsAbnormal tissue growthProguanil Hydrochloride
The invention discloses camphoryl pyrimidines, and a preparation method and an anti-tumor activity study thereof. With camphor as a raw material and under an alkaline catalysis condition, a series ofalpha,beta-unsaturated ketones are obtained through aldol condensation reaction of camphor with different aromatic aldehydes respectively, a series of camphoryl pyrimidines are obtained through annulation reaction of alpha,beta-unsaturated ketones with guanidine hydrochloride through catalysis of potassium tert-butoxide, and the anti-tumor activity of the synthesized camphoryl pyrimidines is studied. Experiments show that the camphoryl pyrimidines have good inhibitory activity on human multiple myeloma cells (RPMI-8226), human breast cancer cells (MDA-MB-231) and human non-small cell lung cancer cells (A549), have less toxicity on normal cell human gastric mucosa cells (GES-1), and have potential anti-tumor application value.
Owner:NANJING FORESTRY UNIV
Enantioselective N-heterocyclic carbene-catalyzed annulation reactions with imidazolidinones
Enantiomeric bicyclic lactone compounds as can be prepared via an N-heterocyclic carbene-catalyzed annulation reaction.
Owner:NORTHWESTERN UNIV
Method for manufacturing section of xenophysogobio boulengeri otolith
InactiveCN103518655AIntuitive and clear wheel pattern featuresImprove accuracyClimate change adaptationPisciculture and aquariaAnnulationEngineering
The invention relates to a method for manufacturing a section of a xenophysogobio boulengeri otolith for fish age determination. The otolith of xenophysogobio boulengeri is picked, soaked, fixed, baked, cooled, grinded, degummed and mounted, clear annulations appear on the otolith of the xenophysogobio boulengeri, and therefore the age of the xenophysogobio boulengeri is determined. The new method is provided for fish age study, and is suitable for the study of the age of teleostean with two sides of the otoliths thick.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Preparation method and application of condensed ring furan-based small-molecular material
InactiveCN106883248AEasy to synthesizeHigh yieldOrganic chemistrySolid-state devicesFuranAcid derivative
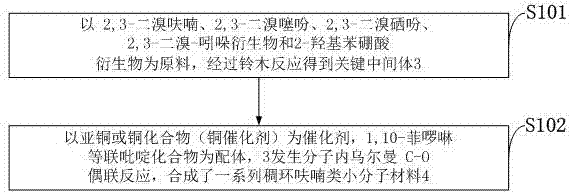
The invention belongs to the technical field of molecular materials and discloses a preparation method and application of a condensed ring furan-based small-molecular material. The method comprises the following steps: by taking 2,3-dibromofuran, 2,3-dibromothiophene, 2,3-dibromoselenophene, 2,3-dibromo-indol derivatives and 2-hydroxybenzeneboronic acid derivatives as raw materials, performing Suzuki reaction to obtain a key intermediate; by taking cuprous or a copper compound as a catalyst, and 1,10-phenanthroline or dipyridyl compound as a ligand, generating intramolecular Ullmann C-O coupling reaction, and synthesizing a series of condensed ring furan small-molecular materials. The substrate is relatively simple to synthesize and high in yield; the ring forming reaction time of furan is greatly shortened; the cuprous compound or dipyridyl compound as a catalyst system are cheap; the reaction conditions are milder; the application range of the substrate is wide; the reaction yield is high up to 97%. Therefore, the method is suitable for industrial production.
Owner:UNIVERSITY OF CHINESE ACADEMY OF SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com