Preparation method and application of condensed ring furan-based small-molecular material
A small molecule and furan technology is applied in the field of preparation of small molecule materials based on fused-ring furan, which can solve the problems of high reaction temperature, low practical value, time-consuming and laborious, and achieve mild reaction conditions, cheap catalytic system, and shortened reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
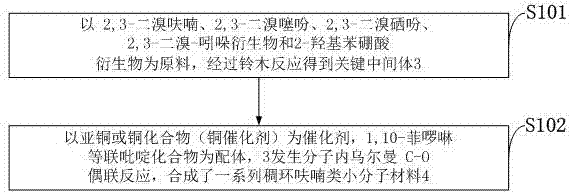
[0036] like figure 1 As shown, the preparation method based on the fused ring furan small molecule material provided by the embodiment of the present invention comprises the following steps:
[0037] S101: Using 2,3-dibromofuran, 2,3-dibromothiophene, 2,3-dibromoselenophene, 2,3-dibromo-indole derivatives and 2-hydroxyphenylboronic acid derivatives as raw materials, The key intermediate 3 was obtained through the Suzuki reaction;
[0038] S102: Using cuprous or copper compounds (copper catalysts) as catalysts, 1,10-phenanthroline and other bipyridine compounds as ligands, 3 undergoes intramolecular Ullmann C-O coupling reaction, and synthesizes a series of fused ring furans Small molecule materials4.
[0039] The synthesis route of the preparation method based on the fused ring furan small molecule material provided by the embodiment of the present invention is as follows:
[0040]
[0041] Wherein Ar and Ar' are selected from any one of aryl, heteroaryl, aryl with subst...
Embodiment 1
[0059] Example 1 Benzo[4,5]thieno[3,2- b ] Synthesis of benzofuran
[0060]
[0061] 1) Accurately weigh 2,3-dibromobenzo[ b ]thiophene (683 mg, 2.34 mmol), 2-hydroxyphenylboronic acid (484 mg, 3.5mmol), and added to a 25 mL Schlenk bottle successively, potassium carbonate (1.29 g, 9.36 mmol), four (three Phenylphosphine) palladium (0.05 equivalent), 1,4-dioxane / water (volume ratio 4:1), placed in an oil bath at 90°C for 6 hours. After the reaction was over, the solvent was removed under reduced pressure, separated using a silica gel column, and petroleum ether / ethyl acetate was used as an eluent to obtain 2-(3-bromobenzo[ b ]thiophen-2-yl)phenol 3a in 84% yield. 1 H NMR (300 MHz, deuterated chloroform): δ 7.86-7.78 (m,2H), 7.47-7.32 (m, 4H), 7.04-6.98 (m, 2H), 5.35 (s, 1H). 13 C NMR (75 MHz, deuterated chloroform): δ 153.42, 138.82, 138.39, 133.55, 131.78, 131.29, 125.96, 125.52, 123.73, 122.45, 120.76, 119.09, 116.44, 108.63. Mass spectrum (EI mode): m / z C 1...
Embodiment 2
[0063] Example 2 Thieno[3,2- b ] Synthesis of benzofuran
[0064]
[0065] 1) Accurately weigh 2,3-dibromothiophene (504 mg, 2.1 mmol), 2-hydroxyphenylboronic acid (433 mg, 3.1 mmol), and add them to a 25 mL Schlenk bottle successively, and add potassium carbonate ( 828 mg, 6 mmol), tetrakis(triphenylphosphine) palladium (0.05 equivalent), 1,4-dioxane / water (volume ratio 4:1), placed in an oil bath at 90°C for 6 hours. After the reaction was over, the solvent was removed under reduced pressure, and the silica gel column was used for separation, and sherwood oil / ethyl acetate was used as eluent to obtain 2-(3-bromobenzene[ b ]thiophen-2-yl)phenol 3b in 87% yield. 1 H NMR (400 MHz, deuterated chloroform): δ 7.43 (d, J = 5.2 Hz, 1H),7.39-7.32 (m, 1H), 7.28 (dd, J = 8.8, 1.2 Hz, 1H), 7.12 (d, J = 5.6 Hz, 1H),7.01-6.98 (m, 2H), 5.13 (s, 1H). 13 C NMR (101 MHz, deuterated chloroform): δ 153.42, 133.16, 131.88, 131.04, 130.96, 127.37, 120.63, 118.80, 116.21, 111.05....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com