Preparation method of allyl chlorooxyl cephalosporin compound
A technology of allyl chloride and compound, applied in the field of pharmaceutical synthesis, can solve problems such as unfavorable industrial production, high cost of iodide, long reaction route, etc., achieve increased steric hindrance, improve yield and purity, and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
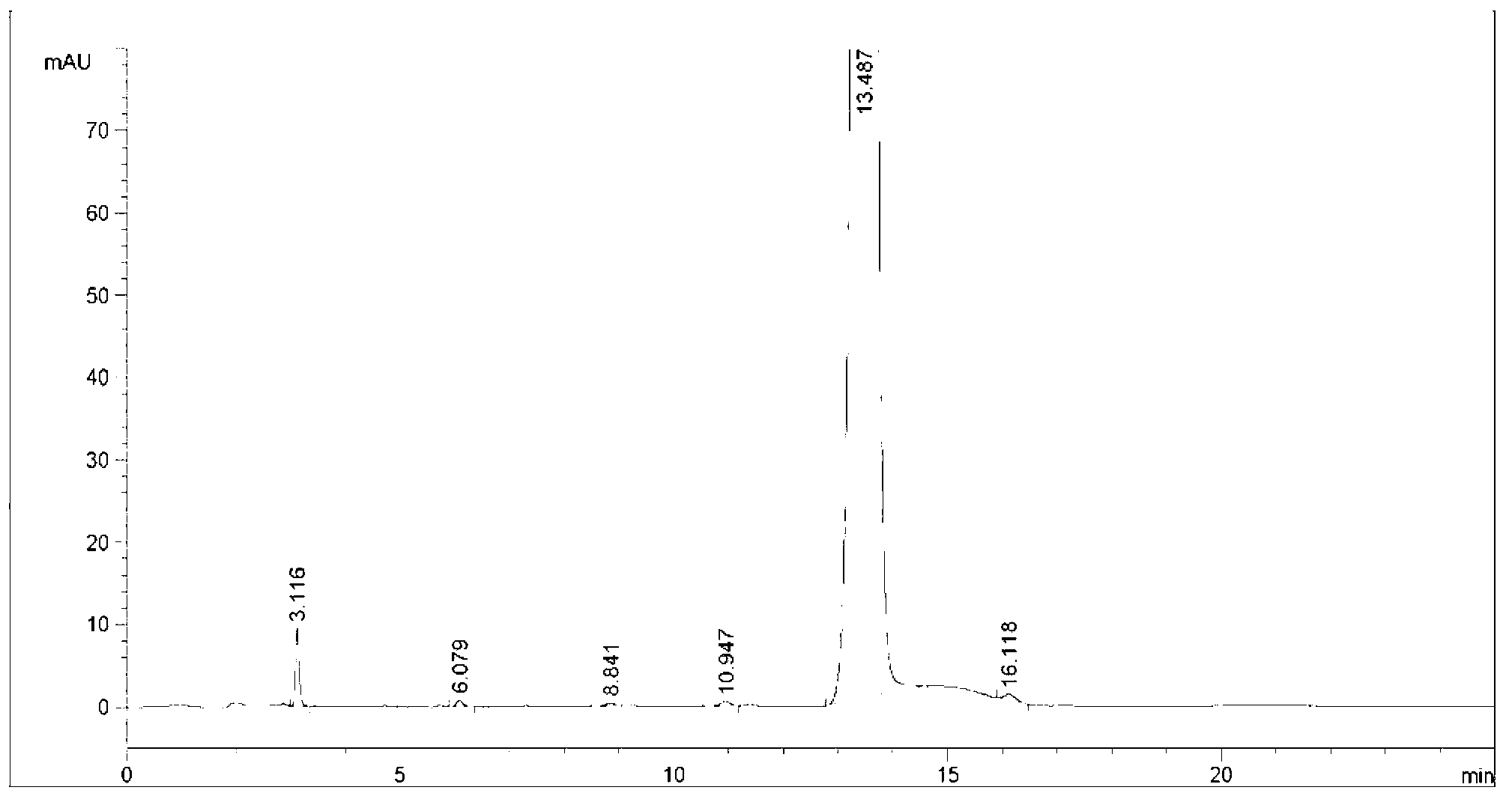
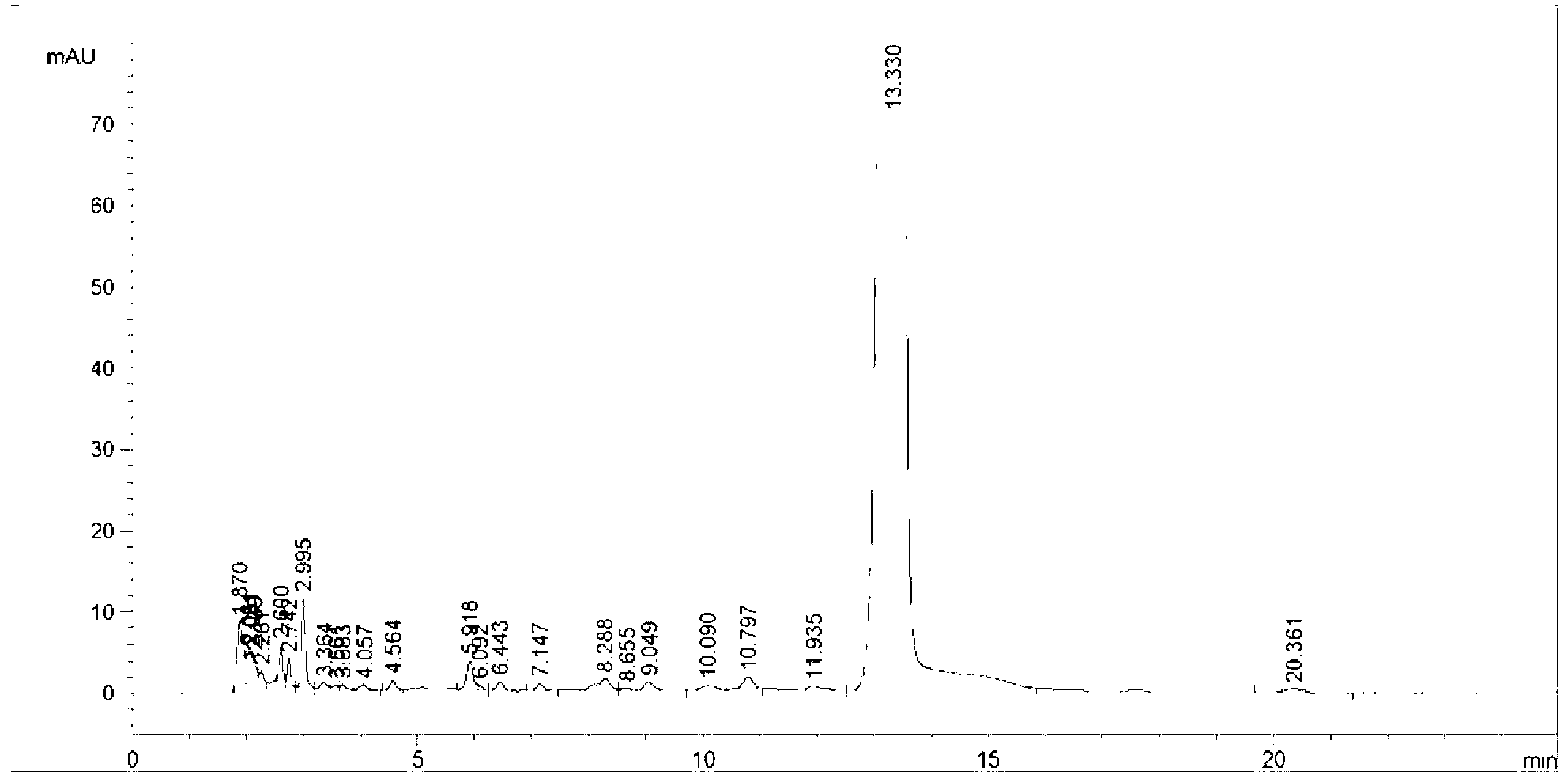
Image
Examples
preparation example Construction
[0046] The preparation method of allyl halooxycephalosporin compound of the present invention specifically comprises the following steps:
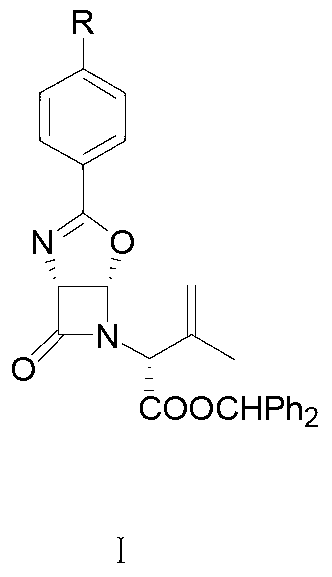
[0047] A. Addition reaction: in a halogenated hydrocarbon solvent, the allyl compound compound of formula I is subjected to an addition reaction with chlorine gas, and after the addition reaction is completed, a reaction solution of the dichloro product compound of formula II is obtained. As a preference, after the addition reaction ends, post-treatment is also included, the post-treatment is specifically: adding a mixed aqueous solution containing sodium sulfite and sodium bicarbonate to the reaction liquid for washing; as a preference, the halogenated hydrocarbon solvent is selected from two One or more of methyl chloride, chloroform, and carbon tetrachloride; the temperature of the addition reaction is -10°C to 0°C, more preferably -5°C to 0°C;
[0048] B. Elimination reaction: Add an organic base to the reaction solution of the compoun...
Embodiment 1
[0053] In a 3000mL three-necked flask, add 93g (0.2mol) of the allyl compound of the formula I compound (R in the compound of the formula I is methyl) 93g (0.2mol), then add 1500mL of dichloromethane to make the solution clear, cool down to -5°C, and control the temperature at -5 ℃ ~ 0 ℃, continuously feed chlorine gas under stirring (control the feeding speed to make it fully absorbed) for addition reaction for 5 hours, take samples, and use TLC (the volume ratio of petroleum ether: ethyl acetate is 2:1 ( mL / mL) is the mobile phase) to carry out follow-up detection to confirm that the reaction is complete. After the addition reaction is completed, the reaction solution of the compound of formula II is obtained; after the addition reaction is completed, post-treatment is also included, and the post-treatment is specifically: to the reaction Add 1000g of aqueous solution containing 50g of sodium sulfite and 60g of sodium bicarbonate to the solution, then, control the temperature...
Embodiment 2
[0059]Add 93g (0.2mol) of the allyl compound of the formula I compound (R in the compound of the formula I is methyl) into a 3000mL three-necked flask, then add 1400mL of chloroform to make the solution clear, cool down to -5°C, and control the temperature at - 5 ℃ ~ 0 ℃, continuously feed chlorine gas under stirring (control the feed rate to make it fully absorbed) for addition reaction for 5 hours, take samples, and use TLC (the volume ratio of petroleum ether: ethyl acetate is 2:1 (mL / mL) of the mobile phase) was tracked and detected to determine that the reaction was complete. After the addition reaction was over, the reaction solution of the compound of formula II was obtained; 1000 g of aqueous solution containing 45 g of sodium sulfite and 50 g of sodium bicarbonate was added to the reaction solution, and then the temperature was controlled. Stir at 8°C for 1.5 hours to wash, let stand, separate layers, collect the organic layer, then add 150mL saturated saline to the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com