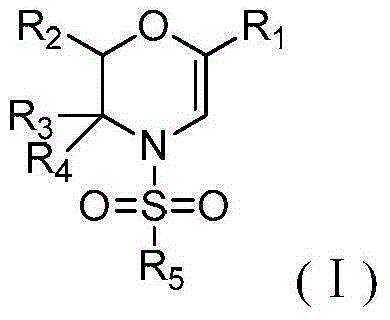
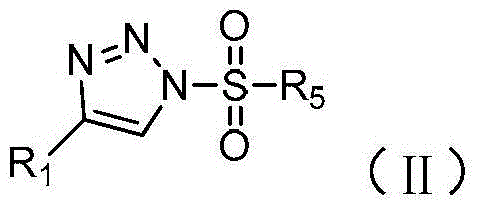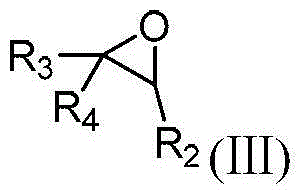Method for preparing N-sulfonyl-1,4-oxazine derivative
A technology of sulfonyl group and derivatives, which is applied in the field of organic synthesis and achieves the effects of simple preparation method, reduced cost, and cheap and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a dry 15mL pressure-resistant reaction tube, add 60mg of N-p-toluenesulfonyl-4-phenyl-1,2,3-triazole (CAS: 884866-01-7), 36mg of styrene oxide, 1.2 mg of dirhodium tetrapivalate and 0.6 ml of 1,2-dichloroethane. The reaction was stirred at 120°C for 1 hour under nitrogen. After the reaction was completed, it was cooled to room temperature, and directly passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 10:1) to obtain 32 mg of product with a yield of 40%. The reaction process is shown in the following formula:
[0029]
[0030] Carry out nuclear magnetic resonance and mass spectrometry to the product prepared in this embodiment:
[0031] 1 H NMR (400MHz, d 6 -acetone): δ=7.81(d,J=8.0Hz,2H),7.53(d,J=8.0Hz,2H),7.44-7.46(m,4H),7.31-7.35(m,4H),7.25- 7.29(m,2H),6.97(d,J=1.2Hz,1H),5.32(s,1H),4.53(dd,J=4.4,2.0Hz,1H),3.28(dd,J=4.4,2.0Hz ,1H),2.43(s,3H);.
[0032]13 C NMR (400MHz, d 6 -acetone): δ=144.3, 140.7, 138.4, 134.5,...
Embodiment 2
[0035] In a dry 15mL pressure-resistant reaction tube, add 63mg of 1-p-toluenesulfonyl-4-(4-methylphenyl)-1,2,3-triazole (CAS: 937801-34-8), 36 mg of styrene oxide, 1.2 mg of dirhodium tetrapivalate and 0.6 ml of 1,2-dichloroethane. The reaction solution was stirred at 120° C. for 1 hour under nitrogen. After the reaction was completed, it was cooled to room temperature, and directly passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 10:1), and 33 mg of the product was obtained, with a yield of 41%. The reaction process is shown in the following formula:
[0036]
[0037] Carry out nuclear magnetic resonance analysis and mass spectrometry to the product prepared in this embodiment:
[0038] 1 H NMR (400MHz, CDCl 3 ): δ=7.67(d, J=4.0Hz, 2H), 7.35-7.38(m, 4H), 7.23-7.29(m, 5H), 7.12(d, J=4.0Hz, 2H), 6.85(d, J=0.4Hz, 1H), 5.00(s, 1H), 4.38(dd, J=5.6, 0.8Hz, 1H), 3.29(dd, J=5.4, 1.4Hz, 1H), 2.41(s, 3H), 2.33(s,3H);
[0039] 13 C ...
Embodiment 3
[0042] In a dry 15mL pressure-resistant reaction tube, add 69mg of 1-p-toluenesulfonyl-4-(1-naphthyl)-1,2,3-triazole (CAS: 1562417-95-1), 36mg of Styrene and 1.2 mg of dirhodium tetrapivalate and 0.6 ml of 1,2-dichloroethane. The reaction solution was stirred at 120° C. for 1 hour under nitrogen. After the reaction was finished, it was cooled to room temperature, and directly passed through a silica gel column (the volume ratio of petroleum ether and ethyl acetate was 10:1), and 30 mg of the product was obtained, with a yield of 37%. The reaction process is shown in the following formula:
[0043]
[0044] Carry out nuclear magnetic resonance and mass spectrometry to the product prepared in this embodiment:
[0045] 1 H NMR (400MHz, CDCl 3 ):δ=7.66(d,J=4.0Hz,2H),7.35(d,J=4.0Hz,2H),7.20-7.28(m,5H),7.08(d,J=4.0Hz,1H),7.00 -7.01(m,1H),6.92(s,1H),6.81(dd,J=4.0,1.2Hz,1H),5.02(s,1H),4.39(dd,J=5.4,0.6Hz,1H), 3.79(s,3H),3.31(dd,J=5.6,0.6Hz,1H),2.41(s,3H);
[0046] 13 C NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com