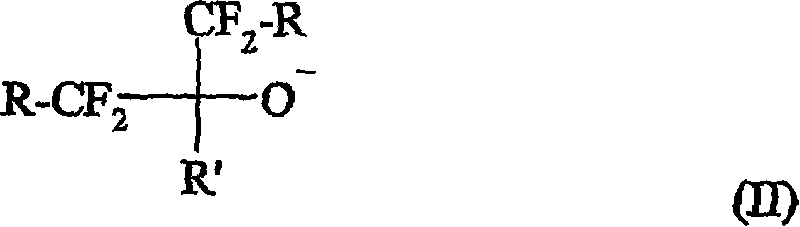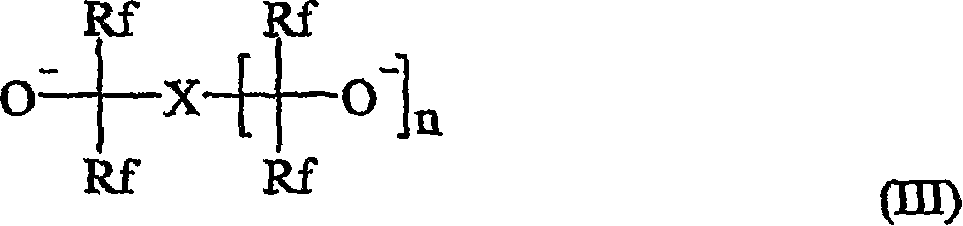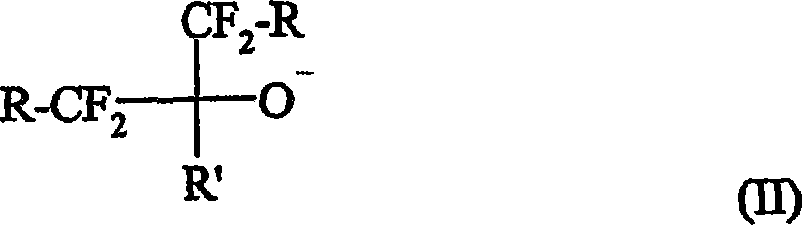Curing compositions for fluoropolymers
A curing agent and polymer technology, applied in the field of fluoropolymer products, can solve the problems of lack of rheological control, unsatisfactory catalyst, opacity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Fluoropolymer A (100 g) was compounded on a two-roll mill with the addition of 2 mmol of SPHI in methanol by adding Prepared by neutralizing 2 mmol PHI in 25% sodium methoxide.
[0153] The resulting compound was pressure cured at 190° C. for 15 minutes (min.) at a level of 2 mmhr. After a post-cure step in air (room temperature to 175°C in 45 minutes, hold at 175°C for 16 hours, then ramp to 300°C in 2 hours and hold at 300°C for 6 hours), The compound obviously formed a triazine cross-linking system (by 1556cm -1 evidenced by the FT-IR peak at ). Physical properties were measured using dumbbell specimens cut from the pressure cured and post cured test pieces. Compression set was measured at a deflection of 25%. The O-ring is completely transparent.
Embodiment 2
[0155] Fluoropolymer A was used in the same operating procedure as in Example 1, except that the curing agent salt used was SHI. The same pressure cure and post cure procedure as in Example 1 was used. After post-curing, at 1556cm -1 A strong triazine signal appeared at the Define the "triazine ratio" as: at 1556cm -1 The triazine peak area at 2200-2700cm -1 The ratio of the area under the C-F pan-spectral band between the two is multiplied by 1000. The "triazine ratio" of the sample prepared in this example was 60.4. The sample is optically clear.
Embodiment 3
[0157] The fluoropolymer A was used in the same operating procedure as in Example 1, except that the curing agent salt used was replaced by cesium ions for sodium ions. The cesium salt was prepared as follows. Dissolve Cs in 1 mL methanol 2 CO 3 (1 mmol, 0.33 g) was slurried, followed by the dropwise addition of PHI (2 mmol, 0.49 g). Gas evolution was seen and the flask warmed slightly, indicating that a reaction had occurred. As in Example 1, this slurry was compounded with 100 grams of the fluoroelastomer compound of Example 1 on a two-roll mill. The same pressure cure and post cure procedure as in Example 1 was used. After the post-curing procedure of Example 1, at 1556 cm -1 A strong triazine signal appeared at the The sample was very pale yellow in color but was still optically clear.
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transparency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com