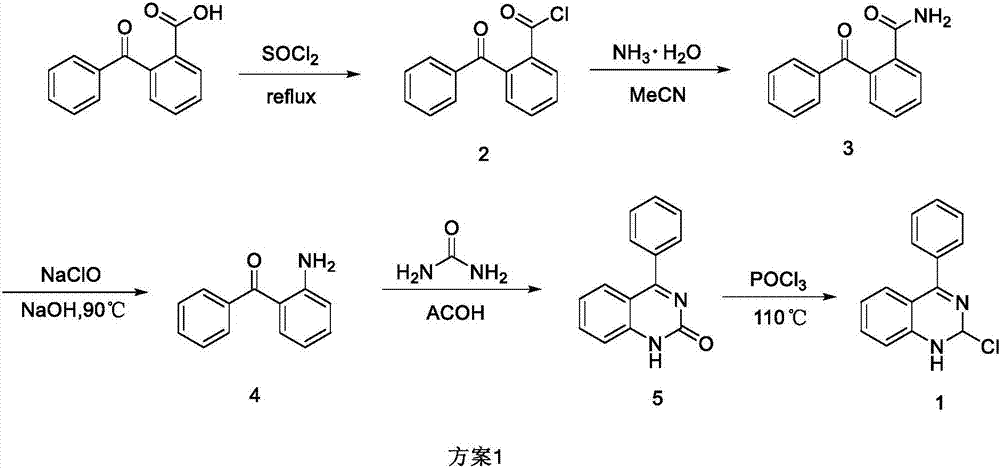
Synthetic method of 2-chloro-4-phenyl quinazoline
A technology of phenylquinazoline and synthesis method, which is applied in the synthesis field of 2-chloro-4-phenylquinazoline, can solve the problems of harsh reaction conditions, high cost, unsuitability for mass production, etc., and achieves simple aftertreatment. , the effect of cheap and easy to obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] (1) Synthesis of o-benzoylbenzoyl chloride 2
[0016] Add o-benzoylbenzoic acid (50 g, 221 mmol (mmol)) into a 500 mL round bottom flask, then add 150 mL of thionyl chloride to dissolve it, and heat to reflux for 1 hour (TLC tracking). After the reaction, the thionyl chloride was evaporated to dryness under reduced pressure at 60°C to obtain 259 g of a brownish-yellow oily substance, which was directly used in the next step without purification.
[0017] (2) Synthesis of o-benzoyl benzamide 3
[0018] Add 150mL of ammonia water into a 500mL three-necked flask, ice bath for 20 minutes, dissolve 2 (59g, 241mmol) in 100mL of acetonitrile, slowly drop into the three-necked flask, and react at room temperature for 1.5h (TLC tracking). After the reaction was completed, it was transferred to a round bottom flask, and the acetonitrile was evaporated to dryness under reduced pressure at 50° C., and a white solid appeared, and then 200 mL of water was added for washing, and suct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com