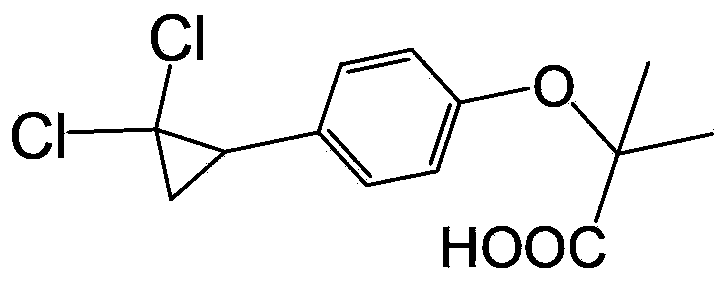
Environment-friendly preparation method for lipid-lowering drug ciprofibrate
A green and pharmaceutical technology, applied to the preparation of organic compounds, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of long reaction route and complicated reaction steps, and achieve high reaction yield, few steps, and easy operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
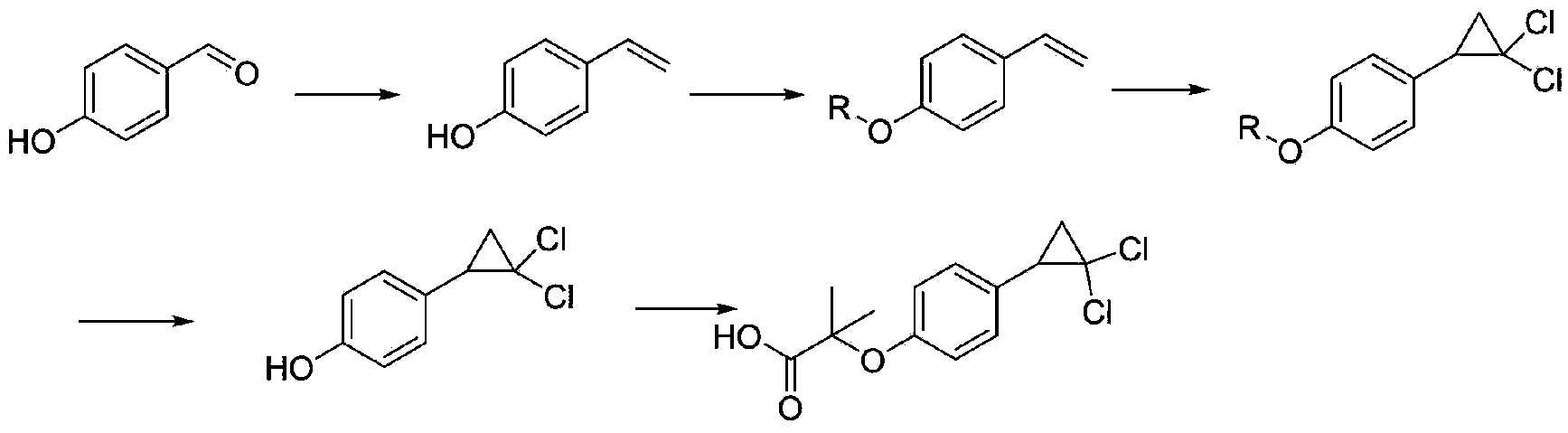
Image
Examples
Embodiment 1
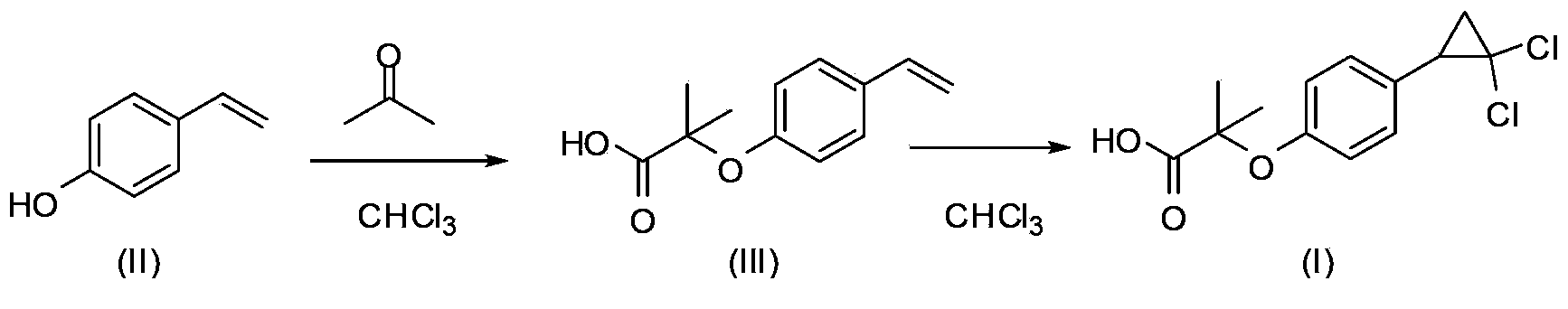
[0030] (1) Preparation of 2-methyl-2-(4-vinylphenoxy)propionic acid
[0031] Add 1kg of p-hydroxystyrene, 3L of acetone, and 1kg of sodium hydroxide into a 10L flask, stir for 30 minutes, then add 1.5kg of chloroform dropwise, stir for 30 minutes after the dropwise addition, and raise the temperature to 70°C. After the reaction is complete, evaporate the solvent to dryness, and add 4000ml Water and 3000ml of dichloromethane, decolorized the water layer with activated carbon, adjusted the pH to 2-3 with 2mol / L hydrochloric acid, precipitated a white product, collected the solid by filtration to obtain 2-methyl-2-(4-vinylphenoxy ) Propionic acid 1.6kg, molar yield 93%. 1 H-NMR (500MHz, CDCl 3 ) δ: 1.65(s,6H), 5.22(d,1H), 5.67(d,1H), 6.69(m,1H), 6.92(d,2H), 7.35(d,2H).
[0032] (2) Preparation of ciprofibrate
[0033] Dissolve 653g of 2-methyl-2-(4-vinylphenoxy)propionic acid in 900ml of chloroform, add 500ml of water, 160g of NaOH, 11g of tetrabutylammonium bromide, stir at r...
Embodiment 2
[0035] (1) Preparation of 2-methyl-2-(4-vinylphenoxy)propionic acid
[0036] Add 1kg of p-hydroxystyrene, 5L of acetone, and 1.8kg of potassium hydroxide into a 10L flask, stir for 30 minutes, then add 2.0kg of chloroform dropwise, stir for 30 minutes after the dropwise addition, control the temperature at 30-40°C, evaporate to dryness after the reaction is complete Solvent, add 5000ml of water and 3000ml of dichloromethane, decolorize the water layer with activated carbon, adjust the pH to 2-3 with 3mol / L hydrochloric acid, precipitate a white product, collect the solid by filtration to obtain 2-methyl-2-(4-ethylene 1.63kg of propionic acid, the molar yield is 94.5%. 1 H-NMR (500MHz, CDCl 3 ) δ: 1.65(s,6H), 5.22(d,1H), 5.67(d,1H), 6.69(m,1H), 6.92(d,2H), 7.35(d,2H).
[0037] (2) Preparation of ciprofibrate
[0038] Dissolve 653g of 2-methyl-2-(4-vinylphenoxy)propionic acid in 1000ml of chloroform, add 500ml of water, 280g of potassium hydroxide, 20g of benzyltriethylammoni...
Embodiment 3
[0040] (1) Preparation of 2-methyl-2-(4-vinylphenoxy)propionic acid
[0041] Add 1kg of p-hydroxystyrene and 5L of acetone to a 10L flask, control the temperature at 0°C, add 3kg of potassium tert-butoxide in batches, stir for 30 minutes, then add 2.0kg of chloroform dropwise at a temperature of 0-10°C, and stir for 30 minutes after the addition , control the temperature at 30-40°C, after the reaction is complete, evaporate the solvent to dryness, add 5000ml of ice water, then add 3000ml of dichloromethane, decolorize the water layer with activated carbon, adjust the pH to 2-3 with 3mol / L hydrochloric acid, and precipitate The white product was collected by filtration to obtain 1.63 kg of 2-methyl-2-(4-vinylphenoxy)propionic acid, with a molar yield of 94.5%. 1 H-NMR (500MHz, CDCl 3 ) δ: 1.65(s,6H), 5.22(d,1H), 5.67(d,1H), 6.69(m,1H), 6.92(d,2H), 7.35(d,2H).
[0042] (2) Preparation of ciprofibrate
[0043]Dissolve 653g of 2-methyl-2-(4-vinylphenoxy)propionic acid in 1000ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com