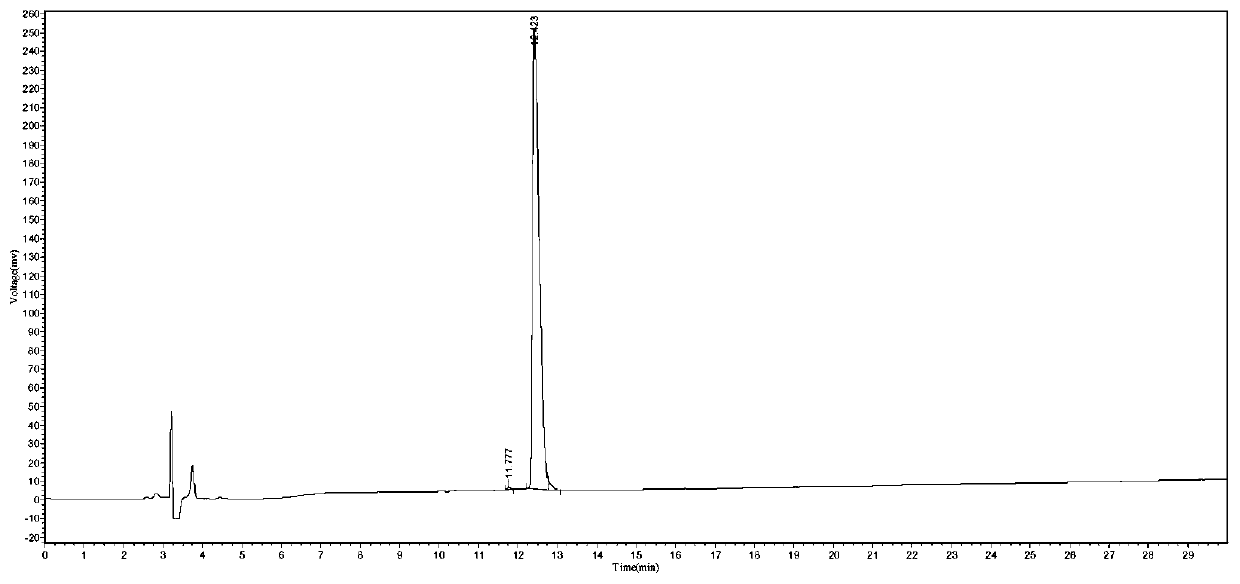
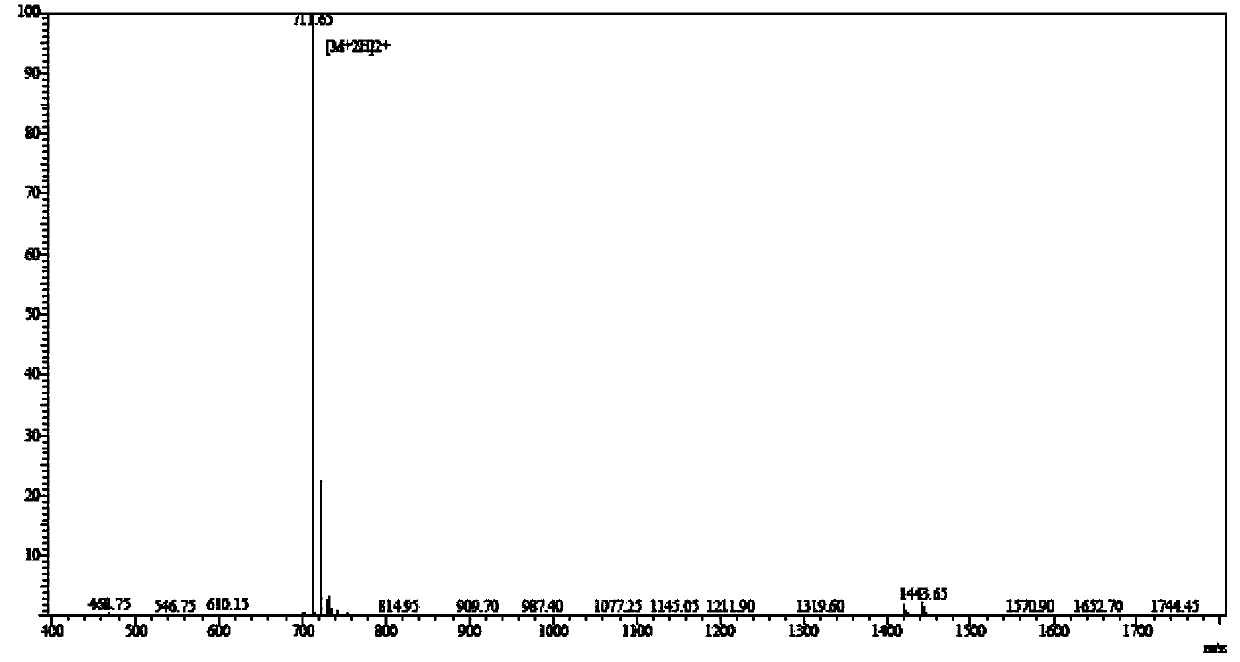
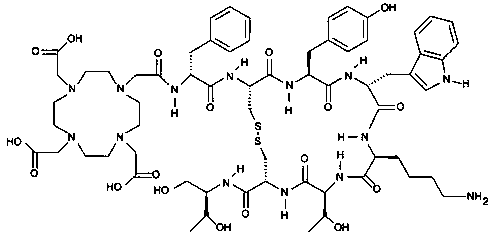
Solid-phase synthesis method for edotreotide
A solid-phase synthesis method and technology of enatreptide, which is applied in the field of solid-phase synthesis of etreptide, can solve problems such as the lack of industrial synthesis methods, and achieve the effect of facilitating later industrial scale-up, less pollution, and greater application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (1) Preparation of Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin
[0058] Weigh 10g of dichlorotriphenyl chloride resin, Fmoc-Thr(tbu)-Ol 3.84g (MW: 383.5, 10mmol), add 180ml of dichloromethane, 20ml of DIEA, react for 2 hours, add 100ml of methanol, react for 30 minutes, filter The filtrate was removed, and the resin was washed 3 times with dichloromethane to obtain Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin;
[0059] (2) Preparation of Enatreptide Resin Peptide I
[0060] Place the Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin in a polypeptide reactor, add 200ml of deprotection reagent, stir and react with nitrogen gas for 30 minutes, drain, wash with DMF 5 times, weigh the protected amino acid, condensing agent, Add it into the reactor, then add 100ml DMF, stir the reaction with nitrogen for 60-120 minutes, detect the end point of the reaction with ninhydrin, drain the reaction solution after the reaction, wash with DMF 3 times, repeat the cycle until the last amino acid is connected, and obtain the ...
Embodiment 2
[0077] (1) Preparation of Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin
[0078] Weigh 10g of dichlorotriphenyl chloride resin, Fmoc-Thr(tbu)-Ol 3.84g (MW: 383.5, 10mmol), add 180ml of dichloromethane, 20ml of DIEA, react for 2 hours, add 100ml of methanol, react for 30 minutes, filter The filtrate was removed, and the resin was washed 3 times with dichloromethane to obtain Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin;
[0079] (2) Preparation of Enatreptide Resin Peptide I
[0080] Place Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin in a polypeptide reactor, add 200ml of deprotection reagent, stir and react with nitrogen gas for 30 minutes, drain, wash with DMF for 5 times, weigh the protected amino acid, condensing agent, Add it into the reactor, then add 100ml DMF, stir the reaction with nitrogen for 60-120 minutes, detect the end point of the reaction with ninhydrin, drain the reaction solution after the reaction, wash with DMF 3 times, repeat the cycle until the last amino acid is connected, and obtain th...
Embodiment 3
[0097] (1) Preparation of Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin
[0098] Weigh 10g of dichlorotriphenyl chloride resin, Fmoc-Thr(tbu)-Ol 3.84g (MW: 383.5, 10mmol), add 180ml of dichloromethane, 20ml of DIEA, react for 2 hours, add 100ml of methanol, react for 30 minutes, filter The filtrate was removed, and the resin was washed 3 times with dichloromethane to obtain Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin;
[0099] (2) Preparation of Enatreptide Resin Peptide I
[0100] Place the Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin in a polypeptide reactor, add 200ml of deprotection reagent, stir and react with nitrogen gas for 30 minutes, drain, wash with DMF 5 times, weigh the protected amino acid, condensing agent, Add it into the reactor, then add 100ml DMF, stir the reaction with nitrogen for 60-120 minutes, detect the end point of the reaction with ninhydrin, drain the reaction solution after the reaction, wash with DMF 3 times, repeat the cycle until the last amino acid is connected, and obtain th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com