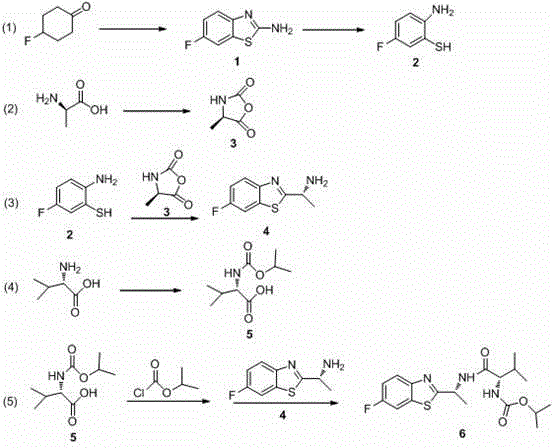
Synthesis technology of benthiavalicarb isopropyl
A technology of benthiazil and synthesis process, applied in the field of pesticide chemistry, can solve the problems of high phosgene, difficult to handle, and long time, and achieve the reduction of heavy metal ion wastewater, less time and material consumption, and less organic solvent consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] (1) Synthesis of 2-amino-6-fluorobenzothiazole (compound 1)
[0023] Add 9 L of dimethyl sulfoxide into a 20 L three-necked flask, add 1 kg (8.62 mol) of p-fluorocyclohexanone, 0.66 kg (8.62 mol) of thiourea, 0.66 kg (2.6 mol) of iodine and 7.4 kg (43 mol) of p-toluenesulfonic acid, then blow in air, heat to control the reaction temperature at about 70 ℃, and react overnight, the system is brownish red or light yellow. After the reaction was completed, water was added to terminate the reaction, extracted with ethyl acetate, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain about 1.36 kg of white solid with a yield of 94%. Melting point 178~180 ℃; 1 H NMR (400 MHz, CDCl 3 ) δ 7.44 (s, 2 H), 7.25 (s, 1 H), 6.03 (d, J = 7.3 Hz, 1H), 5.91 (t, J = 7.6 Hz, 1H); 13 C NMR (100 MHz, CDCl 3 ) δ 176.3, 162.1, 129.4, 126.5, 126.1, 120.7, 118.4.
[0024] (2) Synthesis of 2-amino-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com