Tetra-substituted imidazole synthesis method
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of harsh reaction conditions, large amount of catalyst, poor selectivity, etc., and achieve the effects of being conducive to industrial production, simple process flow, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
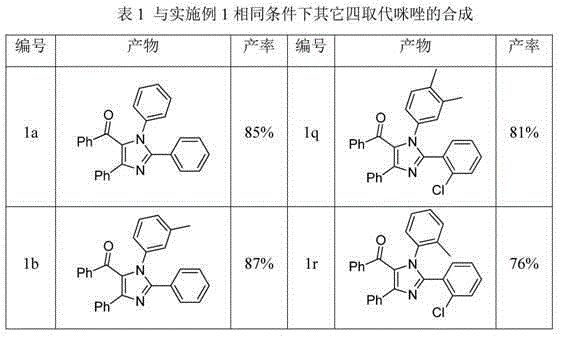
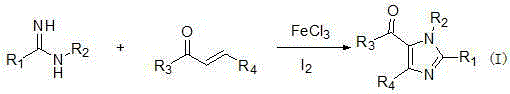
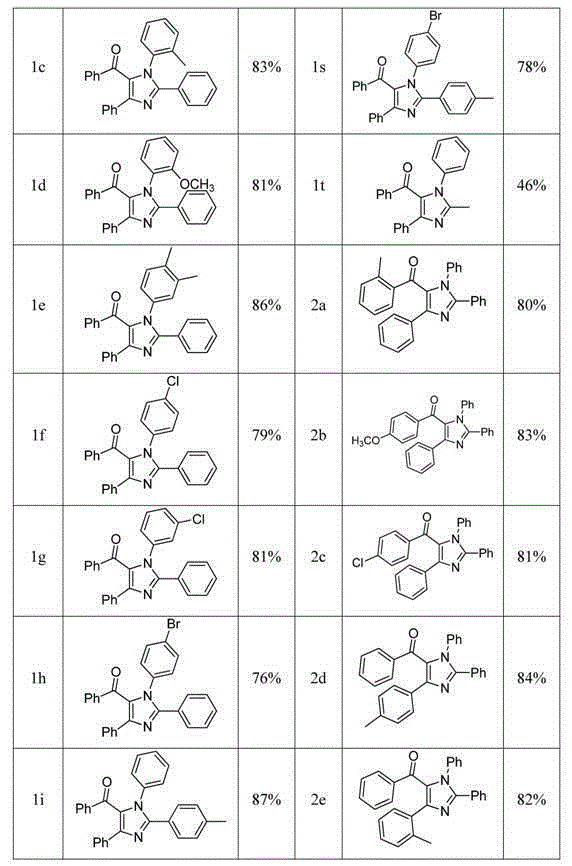
[0019] Example 1 Synthesis of tetrasubstituted imidazoles: take 1a, 1n, 2a as examples to describe in detail.
[0020] Synthesis of 1,2,4-triphenyl-5-benzoyl imidazole (1a)
[0021] Add 0.6mmol N-phenylbenzamidine, 0.5mmol chalcone, 0.01mmol ferric chloride and 0.05mmol iodine into a 25mL round bottom flask containing 2mL chlorobenzene, heat to 110 o C, reacted for 10 h under an oxygen atmosphere. After the reaction (monitored by TLC), it was cooled to room temperature. Iodine was removed with saturated sodium thiosulfate solution, ethyl acetate (15 mL×3) was added three times, then washed with saturated brine, dehydrated with anhydrous sodium sulfate, concentrated and washed with ethyl acetate / petroleum ether ( 1:5) silica gel column chromatography to obtain 170 mg of a yellow solid with a yield of 85%.
[0022] Characterization data: Mp 183?184 °C; 1 H NMR (400 MHz, DMSO) δ 7.68 (t, J = 8.5 Hz, 3H), 7.51 – 7.43 (m, 5H), 7.33 (dd, J = 17.8,10.0 Hz, 4H), 7.21 (dd, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com