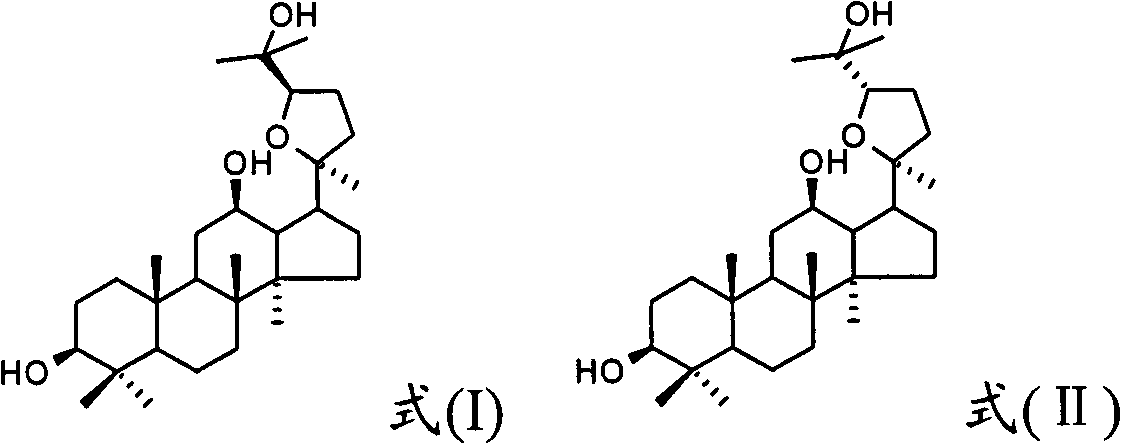
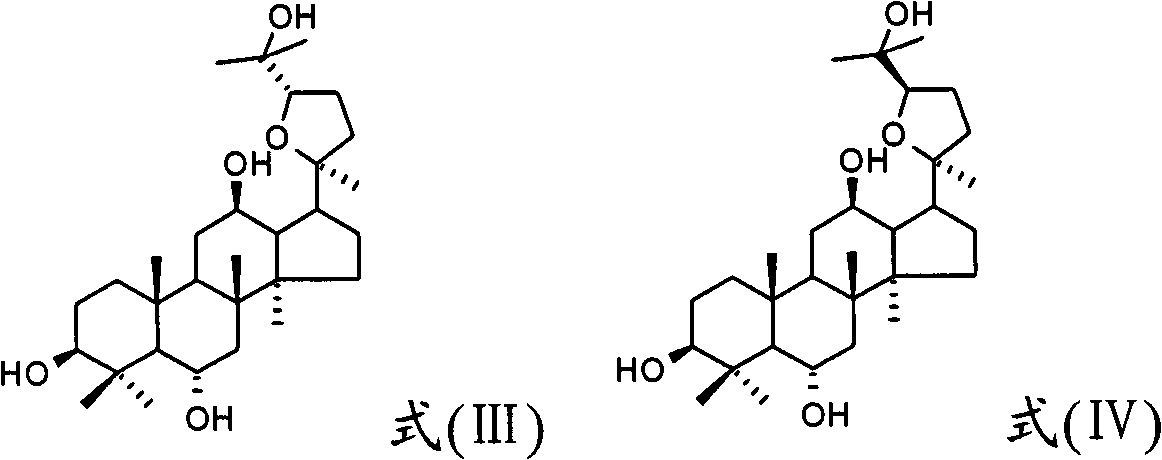
Use of 20(S)-protopanoxadiol derivatives and 20(S)-protopanaxatriol derivatives in preparation of antidepressant medicines
A technology of protopanaxadiol and protopanaxatriol, which can be used in drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve problems such as undisclosed uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] 10g Dama-20S-24(R)-epoxy-3β, 12β, 25-triol, add an appropriate amount of lactose, mix well, use 70% ethanol as a binder, granulate, and put into capsules to obtain capsules , each containing 20 (S)-protopanaxadiol derivatives 20mg.
Embodiment 2
[0099] 10g Dama-20S-24(S)-epoxy-3β, 6β, 12β, 25-tetraol, add an appropriate amount of lactose, mix well, use 70% ethanol as a binder, granulate, and put into capsules to get Capsules, each containing 20(S)-protopanaxadiol derivatives 20mg.
Embodiment 3
[0101] 10g Dama-20S-24(R)-epoxy-3β, 12β, 25-triol, add an appropriate amount of lactose, mix well, use 70% ethanol as a binder, granulate, dry, and add an appropriate amount of magnesium stearate , Tablet compression to obtain tablets, each tablet containing 50 mg of 20(S)-protopanaxadiol derivatives.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com