Application of porphyrin-based covalent organic framework material in photocatalytic oxidation cyclization reaction
A technology of covalent organic framework and photocatalytic oxidation, applied in the photocatalytic oxidative cyclization reaction, based on the field of covalent organic framework materials of porphyrin, can solve the problem that photosensitizers cannot be recycled repeatedly, and the structure synthesis is difficult. Molecules, increase costs and other issues, achieve high universality and repeatability, do not reduce activity, and increase cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] In one embodiment of the present invention, a method for synthesizing N-methyltetrahydro-1H-pyrrolo[3,4-c]-quinoline-1,3(2H)-dione derivatives is provided, The synthetic method comprises the following steps:
[0043] 1) adding N,N-dimethylaniline derivatives and maleimide derivatives into an organic solvent to obtain solution A;
[0044] 2) adding a metal-free covalent organic framework material based on a porphyrin structure as a photocatalyst into solution A to obtain solution B;
[0045] 3) Irradiate solution B with visible light to obtain N-methyltetrahydro-1H-pyrrolo[3,4-c]-quinoline-1,3(2H)-dione derivative.
[0046]
[0047] The technical problem to be solved in this embodiment of the present invention is to use a metal-free covalent organic framework material based on a porphyrin structure as a photocatalyst to catalyze the oxidative cyclization of N,N-dimethylaniline and maleimide through visible light reaction to construct N-methyltetrahydro-1H-pyrrolo[3,...
Embodiment 1
[0063] Synthesis of porphyrin-based covalent organic framework materials H 2 p-Bph-COF:
[0064] Metal-free 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (H 2 TAPP) (32.38 mg, 0.05 mmol) and o-dichlorobenzene / n-butanol (3 / 1 by volume; 1 mL) were passed through three free pump-thaw cycles, and 4, 4-Biphenyldicarbaldehyde (21.02 mg, 0.10 mmol) was degassed; the tube was sealed and heated at 120°C for 3 days. The precipitate was collected by filtration, washed with anhydrous THF and acetone, and dried under vacuum at 120 °C overnight.
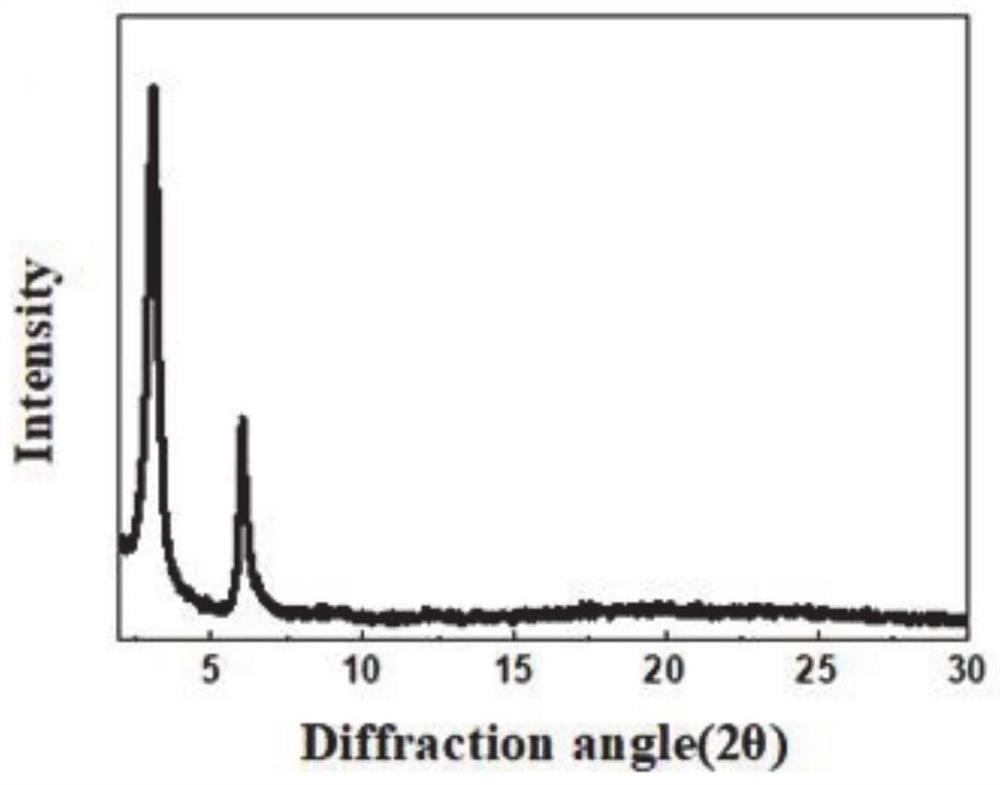
[0065] from figure 1 It can be seen that H 2 p-Bph-COF has good crystallinity, H 2 In the PXRD pattern of p-Bph-COF, the strong diffraction peaks at 3.3° and 7.5° correspond to (1,0,0) and (0,1,0) crystal planes, respectively.
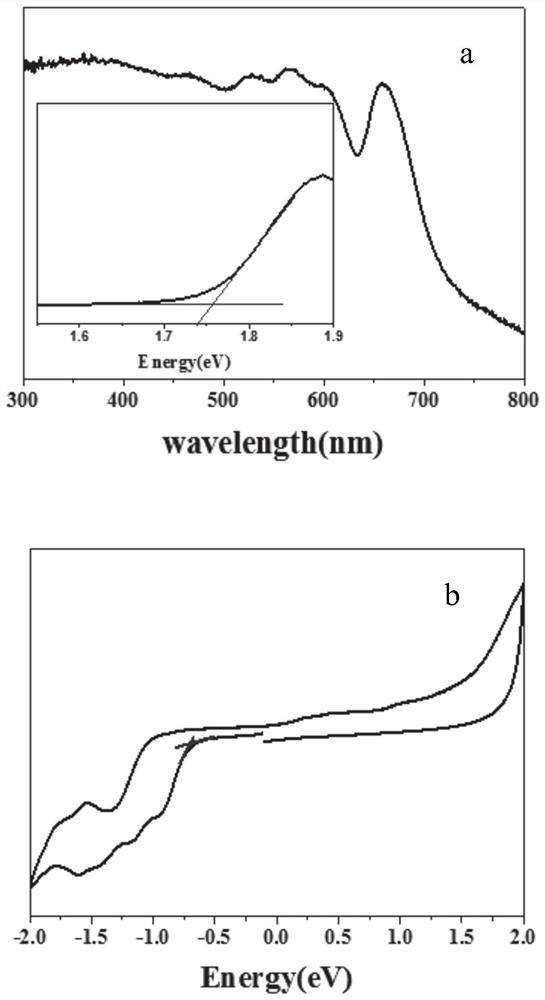
[0066] figure 2 a It can be seen that the absorption of this material is very wide. According to the energy gap conversion formula, the Eg=+1.75eV of the material is obtained, and the figure 2 -b The cyclic voltamm...
Embodiment 2
[0070] h 2 Synthesis of N-methyltetrahydro-1H-pyrrolo[3,4-c]-quinoline-1,3(2H)-dione by oxidative cyclization reaction catalyzed by p-Bph-COF:
[0071] N,N-Dimethylaniline (0.20 mmol, 2.0 eq), maleimide (0.10 mmol, 1.0 eq), H2p-Bph-COF (8 mg, 5 mol%) were placed in a pre-dried In a standard glass reaction tube, chloroform was added as a solvent, irradiated with a blue LED for 14 hours in an oxygen atmosphere, and then the catalyst was collected by centrifugation. The organic layer was separated, and the solvent was removed in vacuo, and purified by silica gel column chromatography (ethyl acetate / petroleum ether=1 / 3, v / v) to obtain the product N-methyltetrahydro-1H-pyrrolo[3,4 -c]-quinoline-1,3(2H)-dione. The yield of product obtained was 70%. H NMR, C NMR, and mass spectrometry identified the product as N-methyltetrahydro-1H-pyrrolo[3,4-c]-quinoline-1,3(2H)-dione.
[0072] The hydrogen spectrum and carbon spectrum of the product N-methyltetrahydro-1H-pyrrolo[3,4-c]-quinoli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com