Synthesis method of geosmin
A synthetic method, the technology of geosmin, which is applied in the direction of organic chemistry, oxygen-containing functional group reduction preparation, etc., can solve the problems of risk, high cost, difficulty in obtaining raw materials, etc., to ensure product purity and yield, and reduce preparation Effect of less cost and solvent usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
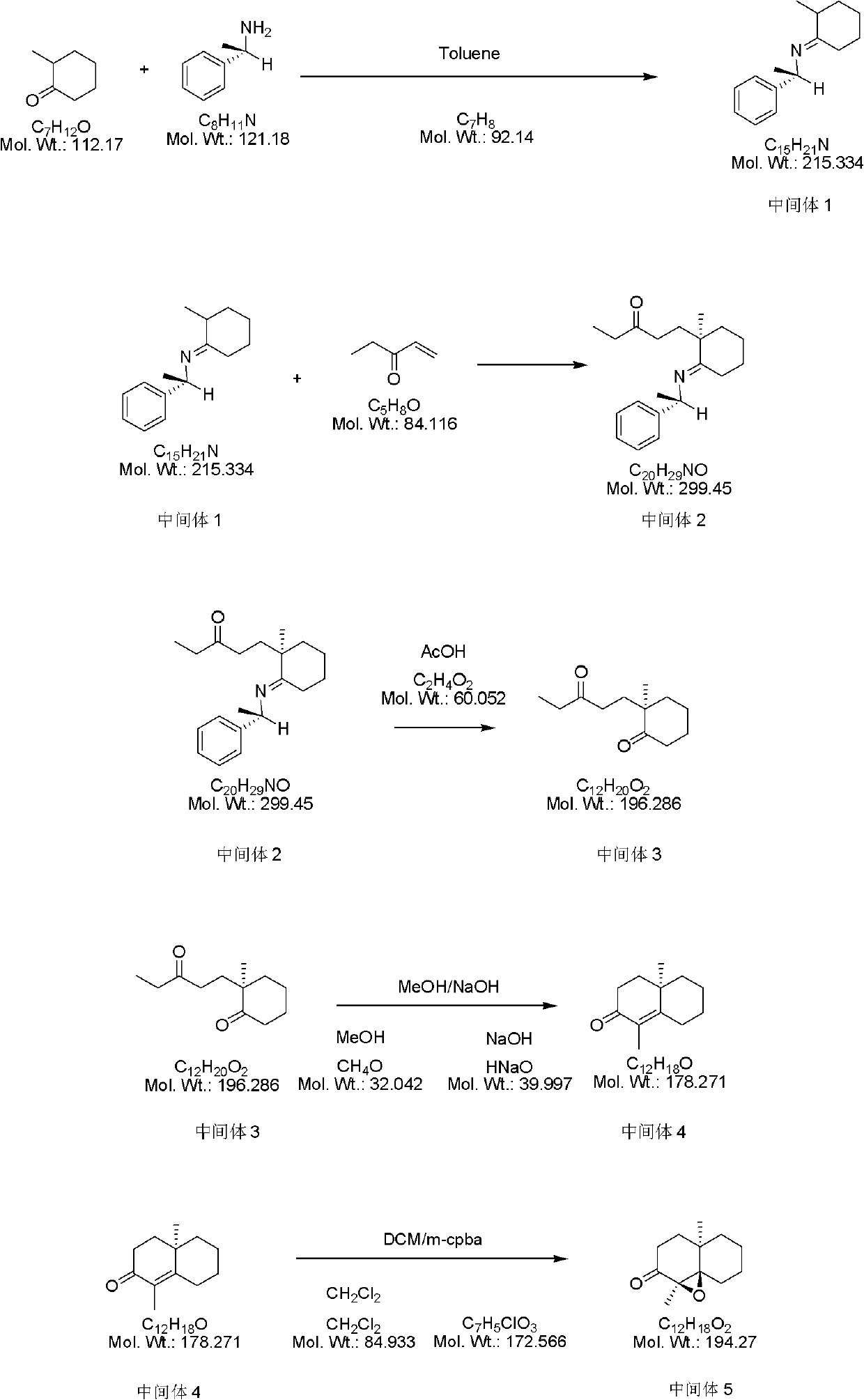
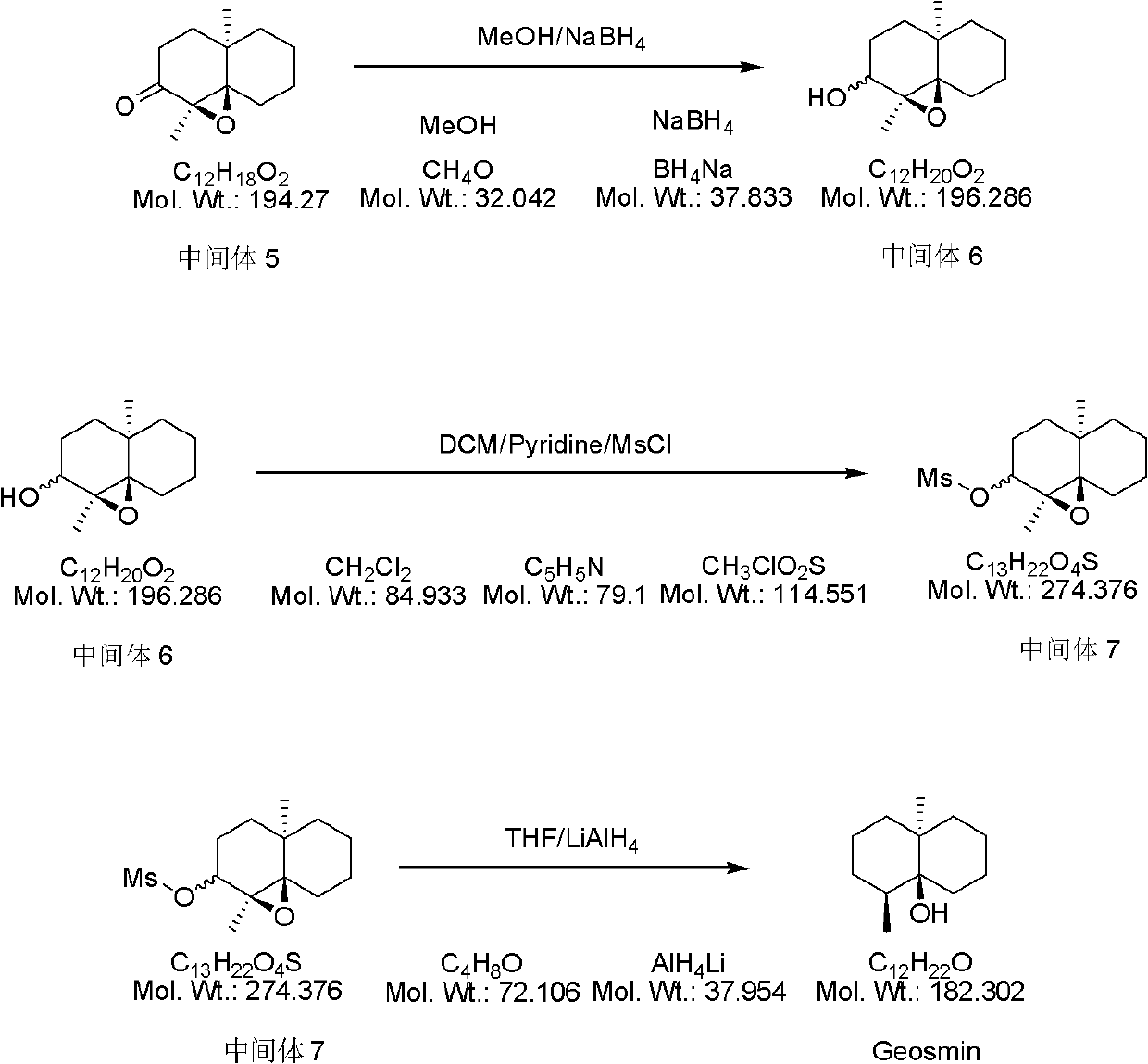
[0028] 1) Add 9 grams of 2-methylcyclohexanone into a four-necked flask, then add 40 grams of toluene and 13 grams of (S)-α-phenylethylamine in turn at room temperature. After the addition is complete, the above reaction solution Heat to reflux and stir for 6 hours, stop stirring, distill the toluene to obtain the crude product, and distill the crude product under reduced pressure to obtain 13 g of intermediate 1, yield: 75%.
[0029] 2) Mix 13 g of intermediate 1 and 5.3 g of pentenone at room temperature, then stir the above reaction solution at room temperature for 24 hours, stop stirring to obtain 18.3 g of intermediate 2, yield: 100%.
[0030] 3) Mix 18.3 grams of intermediate 2 with 4 grams of glacial acetic acid at room temperature, then stir the above reaction solution at room temperature for 1 hour, add 30 grams of ethyl acetate for dilution, and wash the resulting solution with saturated sodium bicarbonate solution and water until The aqueous phase was neutral, and t...
Embodiment 2
[0037] 1) Add 13.5 grams of 2-methylcyclohexanone into a four-necked flask, then add 55 grams of toluene and 16.2 grams of (S)-α-phenylethylamine in turn at room temperature, and after the addition is complete, the above reaction solution Heat to reflux and stir for 10 hours, stop the stirring, distill off the toluene to obtain a crude product, which is distilled under reduced pressure to obtain 20.5 g of intermediate 1, yield: 79%.
[0038] 2) 20.5 g of intermediate 1 and 8.8 g of pentenone were mixed at room temperature. After the mixing, the above reaction solution was stirred at room temperature for 30 hours, and the stirring was stopped to obtain 29.3 g of intermediate 2. The yield: 100%.
[0039] 3) Mix 29.3 grams of intermediate 2 with 9 grams of glacial acetic acid at room temperature, stir the above reaction solution at room temperature for 3 hours, add 40 grams of ethyl acetate to dilute, and wash the resulting solution with saturated sodium bicarbonate solution and w...
Embodiment 3
[0046] 1) Add 18.9 grams of 2-methylcyclohexanone into a four-necked flask, then add 90 grams of toluene and 41 grams of (S)-α-phenylethylamine in turn at room temperature, and after the addition is complete, the above reaction solution Heat to reflux and stir for 10 hours, stop stirring, distill off the toluene to obtain a crude product, which is distilled under reduced pressure to obtain 29.4 g of intermediate 1, yield: 81%.
[0047] 2) 29.4 g of intermediate 1 and 14.7 g of pentenone were mixed at room temperature. After the mixing was completed, the above reaction solution was stirred at room temperature for 48 hours, and the stirring was stopped to obtain 44.1 g of intermediate 2, yield: 100%.
[0048] 3) Mix 44.1 grams of intermediate 2 with 9 grams of glacial acetic acid at room temperature, stir the above reaction solution at room temperature for 5 hours, add 15 grams of ethyl acetate to dilute, and wash the resulting solution with saturated sodium bicarbonate solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com