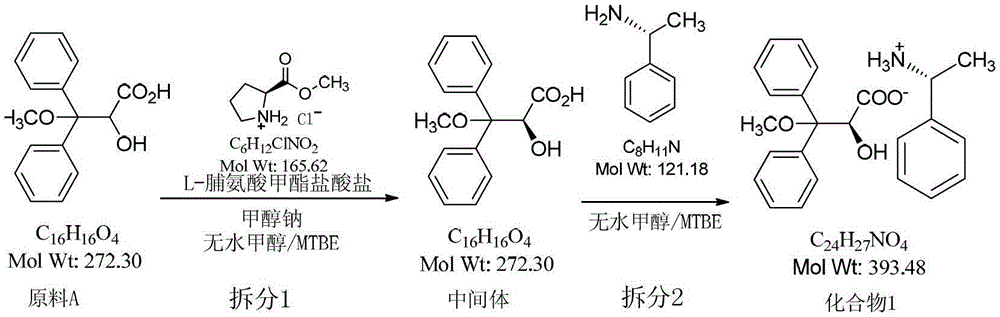
Resolution method of 2-hydroxy-3-methoxy-3,3-dibenzylpropionic acid racemate
A technology of diphenylpropionic acid and methoxy, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds. problem, to achieve the effect of strong reproducibility and easy industrial implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add L-proline methyl ester hydrochloride (6.5kg, 39.25mol) and anhydrous methanol (3.5L) into a 10L reaction flask, stir well, then add sodium methoxide (2.12kg, 39.25mol) in batches Anhydrous methanol (5.0L) mixture, after the addition, stirred and reacted at (15-30°C) for 20-30min, filled the mixed solution into a 300L reactor, and then filled the raw material A (10.68kg, 39.25mol) The methyl tert-butyl ether (90L) solution is also filled into the above reaction kettle, the temperature is controlled at (15-30°C) and the reaction is stirred for 20 hours, and the methyl tert-butyl ether (180L) is added to the reaction kettle, and the temperature is reduced by circulation. Control the internal temperature at -5~0°C and stir for 30 minutes, filter, transfer the filtrate to a 500L reaction kettle, add 3.5L of hydrochloric acid and 130L of purified water while stirring, stir for 3~5min, let stand for 10~15min, discard the water layer, organic The layer was dried with anhydr...
Embodiment 2
[0031] Add L-proline methyl ester hydrochloride (6.5kg, 39.25mol) and anhydrous methanol (3.5L) into a 10L reaction flask, stir well, then add sodium methoxide (2.12kg, 39.25mol) in batches Anhydrous methanol (5.0L) mixture, after the addition, stirred and reacted at (15-30°C) for 20-30min, filled the mixture into a 300L reactor, and then filled the raw material A (9.72kg, 35.68mol) The methyl tert-butyl ether (90L) solution is also filled into the above reaction kettle, the temperature is controlled at (15-30°C) and the reaction is stirred for 20 hours, and the methyl tert-butyl ether (180L) is added to the reaction kettle, and the temperature is reduced by circulation. Control the internal temperature at -5~0°C and stir for 30 minutes, filter, transfer the filtrate to a 500L reactor, add 3.5L of hydrochloric acid and 130L of purified water while stirring, stir for 3~5min, let stand for 10~15min, discard the water layer, organic The layer was dried with anhydrous magnesium su...
Embodiment 3
[0033] Add raw material A (11kg, 40.4mol), anhydrous methanol (51L), methyl tert-butyl ether (51L) into a 200L reactor, drop (R)-(+)-α- Phenylethylamine (4.4kg, 36.3mol), after dropping, stirred at 10-30°C for 3-4h, filtered, washed the filter cake with methyl tert-butyl ether (12L×2), dried to obtain 3.55kg, yield 64.5 %. HPLC purity: 99.5%, chiral purity: 68.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com