Zinc nitride and copper nitride compound of chiral alpha-phenylethylamine and use thereof
A technology of copper-nitrogen complexes and phenylethylamine, which is applied in the direction of copper organic compounds, zinc organic compounds, and preparation of amino compounds from amines, etc., which can solve the problems of unstable catalysts, difficult industrialization, and large amount of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
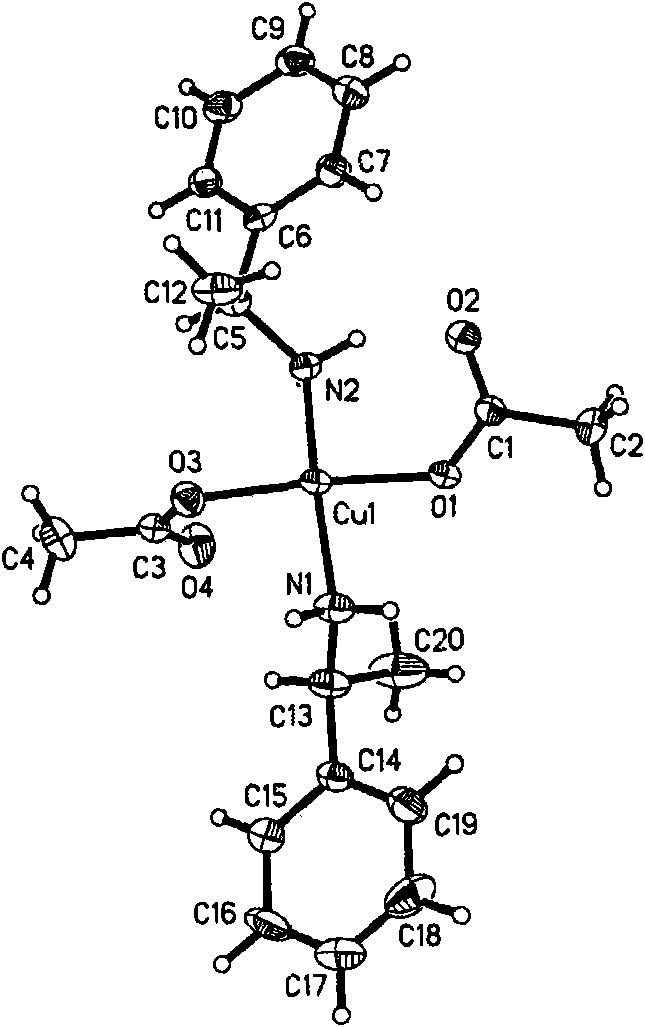
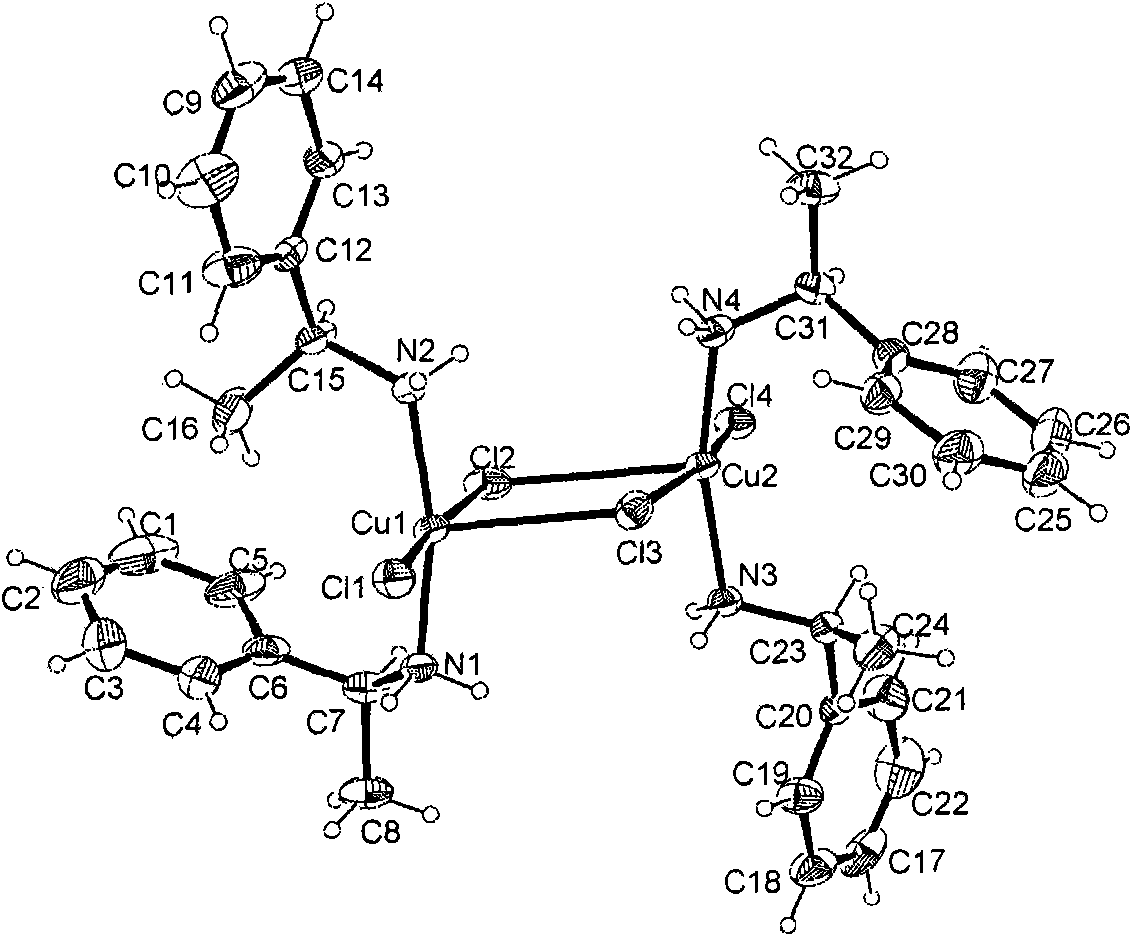
[0075] (1) Examples of preparation of chiral complexes
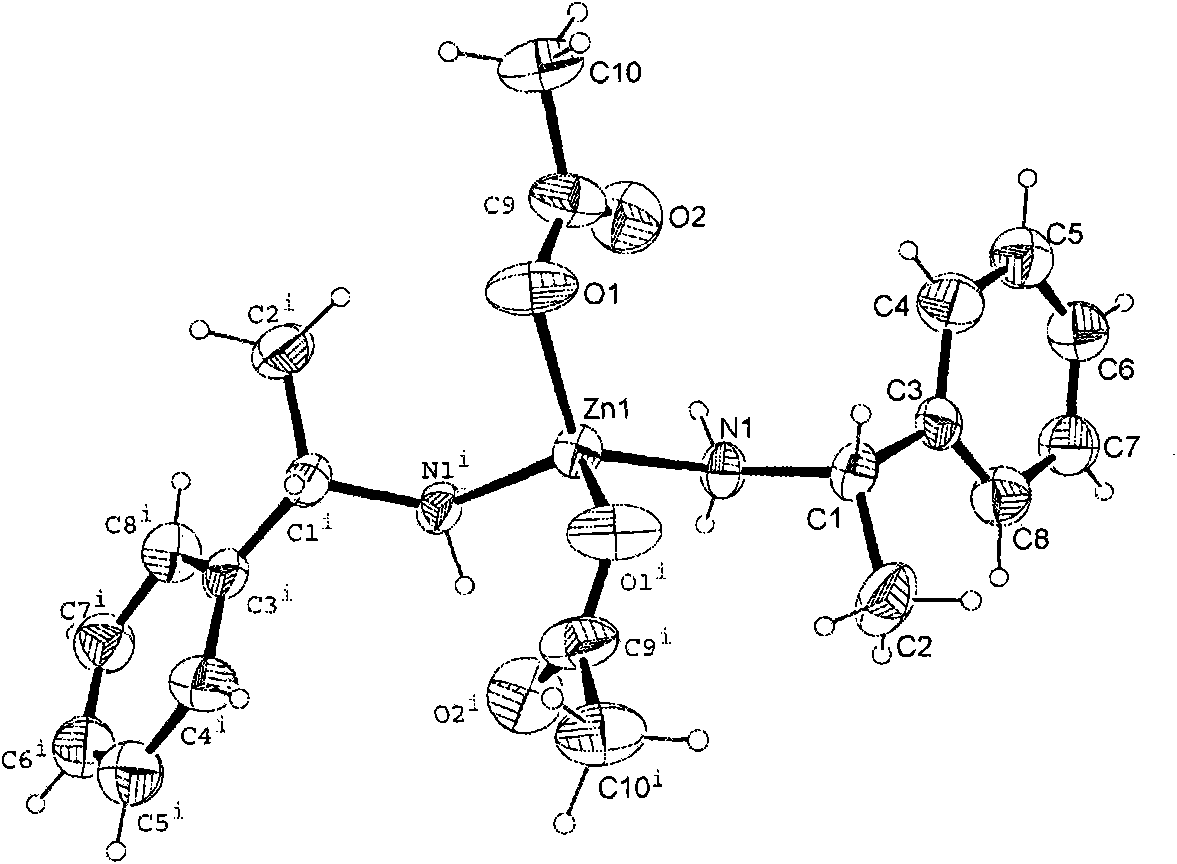
[0076] 1, 1a: Synthesis of chiral (S)-α-phenylethylamine zinc acetate metal-organic complexes
[0077] Weigh 2.191g Zn(CH 3 COO) 2 2H 2 O (0.01mol) was added to a 100mL round-bottomed flask, and then 25mL of tetrahydrofuran was added as a solvent, a stirring bar was added, and 2.7mL (S)-α-phenylethylamine (0.02mol) was measured with a syringe under stirring and added to In the flask, install a condensing tube, connect tap water, place on a magnetic heating stirrer and heat to reflux for 24 hours, remove the solvent with a rotary evaporator, recrystallize with a mixed solvent of petroleum ether and absolute ethanol (mass ratio 1: 1), Colorless crystals were obtained with a yield of 90%. 1 HNMR (500MHz, CDCl 3 )7.22~7.31(m, 10H), 4.06~4.07(d, J=6.5Hz, 1H), 3.06(br, 1H), 1.94(s, 6H), 1.40~1.42(d, J=6.5Hz, 6H ). 13 C NMR (75MHz, CDCl 3 )179.70, 144.04, 128.94, 127.92, 125.92, 51.82, 23.94, 23.10. IR (KBr) cm -1 : 31...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com