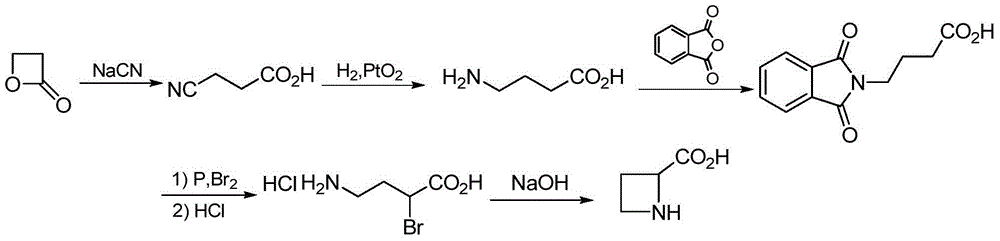
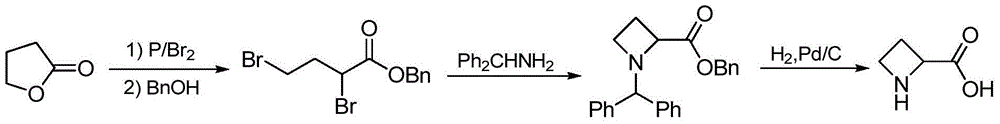
The preparation method of (s)-azetidine-2-carboxylic acid
A technology for azetidine and tetracyclobutane, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as difficult and low-cost target compounds, and achieves the effects of low cost of raw materials, improved splitting effect and short synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Heat 500g of 1-benzylcyclobutane-2-carboxylic acid (synthesized according to Tetrahedron:Asymmetry, 1998, 9:429-435) in 3L of ethanol, then add 200g of D-α-phenylethylamine dropwise to form a salt, After dripping, continue to reflux for 0.5 hours. Then slowly lower to room temperature, stand still, precipitate crystals and filter, dissolve in 1L of water, then adjust the pH value to 10 with 10% NaOH solution, extract and recover D-α-phenylethylamine with dichloromethane; Adjust the pH value to 2 with hydrochloric acid, then extract with ethyl acetate, spin dry the solvent, and recrystallize the obtained solid with ethanol to obtain 189 g of white crystals (S)-1-benzylazetidine-2-carboxylic acid, HPLC>99%, ee>98%, yield 37.8%.
[0034] 1 H NMR (D 2 O,500MHz):δ2.39-2.43(m,1H,Aze-CH 2 ),2.60-2.64(m,1H,Aze-CH 2 ),3.81-3.85(m,1H,Aze-CH 2 ),3.96-4.00(m,1H,Aze-CH 2 ),3.3(q,J=13Hz,2H,Bn-CH 2 ), 4.727 (t, J=9.5Hz, 1H, Aze-CH), 7.408-7.412 (m, 5H, Bz-H).
[0035] Add 150...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com