Method for catalytically synthesizing L-cis-1,2-epoxypropylphosphonic acid-D-alpha-phenylethylamine by using phosphotungstic heteropoly acid phase transfer catalyst
A phase transfer catalyst, the technology of levophosphorus dexamine salt, which is applied in chemical instruments and methods, preparation of amino compounds from amines, compounds of elements of Group 5/15 of the periodic table, etc., can solve the problem that the catalyst cannot be recycled and restricts the production cost of products and other problems, to achieve the effect of high recovery rate, high catalytic activity and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
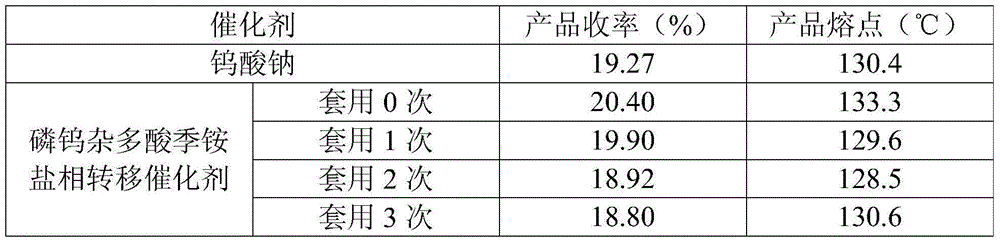
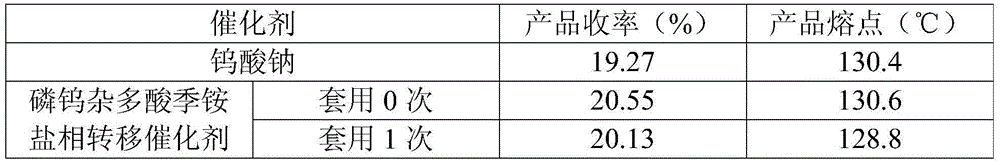
[0009] In a three-neck flask equipped with a magnetic stirrer and a thermometer, add 3.0 g of crude cis-acrylphosphonic acid and 10 mL of ethanol with a mass concentration of 95%, and stir to dissolve at room temperature. Control the temperature in the water bath to 30-40°C, slowly add α-phenylethylamine dropwise, adjust the pH=5.5-6.0, and continue stirring for 5 minutes after dropping. Add 0.1714g of catalyst wet product (containing about 30wt% ethanol) and 0.03g of EDTA, heat to 40-50°C, slowly add 3.065g of hydrogen peroxide solution with a mass concentration of 30%, after the dropwise addition, keep the temperature at 50°C for reaction, 1 HNMR detection reaction. After the reaction is completed, cool down to -5~-10°C, keep warm for 0.5h, filter with suction, wash the filter cake with ethanol at 0~-5°C, and refine it to obtain Zuo salt ( 1 HNMR confirmed the structure: (1R,2S)-(-)-cis-1,2-epoxypropyl phosphate-R-(+)-α-phenethylamine salt). The filtrate was raised to room...
Embodiment 2
[0013] In a three-neck flask equipped with a magnetic stirrer and a thermometer, add 3.0 g of crude cis-acrylphosphonic acid and 10 mL of ethanol with a mass concentration of 95%, and stir to dissolve at room temperature. Control the temperature in the water bath to 30-40°C, slowly add α-phenylethylamine dropwise, adjust the pH=5.5-6.0, and continue stirring for 5 minutes after dropping. Add 0.0857g catalyst wet product (containing about 30wt% ethanol) and 0.03g EDTA, heat to 40-50°C, slowly add 3.345g of hydrogen peroxide solution with a mass concentration of 30%, after the dropwise addition, keep the temperature at 50°C for reaction, 1 HNMR detection reaction. After the reaction is completed, cool down to -5~-10°C, keep warm for 0.5h, filter with suction, wash the filter cake with ethanol at 0~-5°C, and refine it to obtain Zuo salt ( 1 HNMR confirmed the structure: (1R,2S)-(-)-cis-1,2-epoxypropyl phosphate-R-(+)-α-phenethylamine salt). The filtrate was raised to room tempe...
Embodiment 3
[0018] In a three-neck flask equipped with a magnetic stirrer and a thermometer, add 3.0 g of crude cis-acrylphosphonic acid and 10 mL of ethanol with a mass concentration of 95%, and stir to dissolve at room temperature. Control the temperature in the water bath to 30-40°C, slowly add α-phenylethylamine dropwise, adjust the pH=5.5-6.0, and continue stirring for 5 minutes after dropping. Add 0.1714g of catalyst wet product (containing about 30wt% ethanol) and 0.03g of EDTA, heat to 40-50°C, slowly add 3.065g of hydrogen peroxide solution with a mass concentration of 30%, after the dropwise addition, keep the temperature at 50°C for reaction, 1 HNMR detection reaction. After the reaction is completed, cool down to -5~-10°C, keep warm for 0.5h, filter with suction, wash the filter cake with ethanol at 0~-5°C, and refine it to obtain Zuo salt ( 1 HNMR confirmed the structure: (1R,2S)-(-)-cis-1,2-epoxypropyl phosphate-R-(+)-α-phenethylamine salt). The filtrate was raised to room...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com