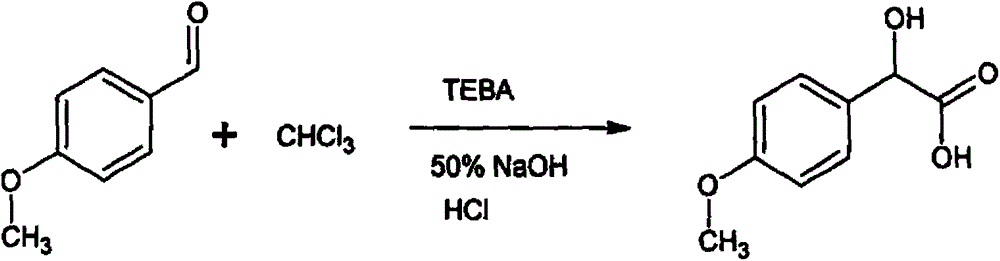
Resolving synthetic method for paramethoxymandelic acid
A technology of p-methoxymandelic acid and methoxymandelic acid salt, which is applied in the field of synthesis and resolution of p-methoxymandelic acid, can solve the problems of difficult separation and purification of products, strong corrosion of halogenated reagents, etc., and achieve Ease of industrial production, easy product separation, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Synthesis of DL-p-methoxymandelic acid
[0017] Add 272 grams of p-methoxybenzaldehyde, 1,500 milliliters of chloroform, and 54.4 g of benzyltriethylammonium chloride into a 5-liter reaction flask with heating and reflux, raise the temperature to 35-45 ° C, and start dripping under sealed conditions. Add 750 ml of 50% NaOH, react for 12 hours, and the reaction is over. After adding water to dissolve, adjust the pH to 1 with concentrated hydrochloric acid, then extract with ethyl acetate, combine the ethyl acetate phases, wash with saturated brine, dry the organic phase, and decolorize , concentrated, added petroleum ether to crystallize. Obtained 256 grams of white solid DL-p-methoxymandelic acid, yield 70%, MP: 101-103 °C;
[0018] (2) Synthesis of L-p-methoxymandelic acid·S-α-phenethylamine salt
[0019] Add 200G of p-methoxymandelic acid, isopropanol: water 2L (volume ratio 9:1) to a 5-liter reaction flask, then heat up to 40-70°C, add S-phenylethylamine 150G in...
Embodiment 2
[0023] (1) Synthesis of D-p-methoxymandelic acid·R-α-phenethylamine salt
[0024] Add in 5 liters of reaction bottles, DL-p-methoxymandelic acid 200G, isopropanol: water 1800 milliliters (volume ratio 9: 1) then warm up to 40-60 ℃ and add R-phenylethylamine 136G in batches, then 70°C heat preservation reaction for 3 hours, then put it in an ice bath, filter after about 0°C, rinse the filter cake with a small amount of isopropanol: water to obtain coarse salt, and then use isopropanol: water (volume ratio 9 : 1) After heating and dissolving, take an ice-water bath, filter after the solid is precipitated, wash with a small amount of isopropanol: water, and repeat recrystallization 3 times to finally obtain 60 grams of pure D-p-methoxymandelic acid·-α-phenylethylamine salt. Optical rotation: -60°C=1 water.
[0025] (2) Synthesis of L-p-methoxymandelic acid
[0026] Add 60 grams of L-p-methoxymandelic acid S-α-phenylethylamine salt, add 180 ml of water, 180 ml of methanol, then ...
Embodiment 3
[0028] A method for the resolution and synthesis of p-methoxymandelic acid, the method comprising the following steps:
[0029] (1) Under alkaline conditions, p-methoxybenzaldehyde, chloroform, and benzyltriethylammonium chloride (TEBA) were reacted at 35-45°C for 12 hours, extracted by acidification, and crystallized to obtain racemic p- Methoxymandelic acid. The mass ratio of described p-methoxybenzaldehyde and benzyltriethylammonium chloride is 5: 1, and the mass-volume ratio of described p-methoxybenzaldehyde and chloroform is 0.1: 1g / L; The alkaline condition is under the action of 50% sodium hydroxide.
[0030] (2) Using chiral α-phenylethylamine as a resolving agent, p-methoxymandelic acid and chiral α-phenylethylamine are carried out in a mixed solvent with a molar ratio of 1: 1.0 to carry out a salt-forming reaction. The mixed solvent is formed by mixing isopropanol and water in a volume ratio of 9:1; the weight-to-volume ratio of the p-methoxymandelic acid to the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com