A kind of synthetic method of ubenimex
A synthesis method, the technology of ubimethox, is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of long recrystallization time, complicated synthesis steps, long reaction time, etc., and achieve intermediate products. Fewer, Simplified Steps, Simple Step Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
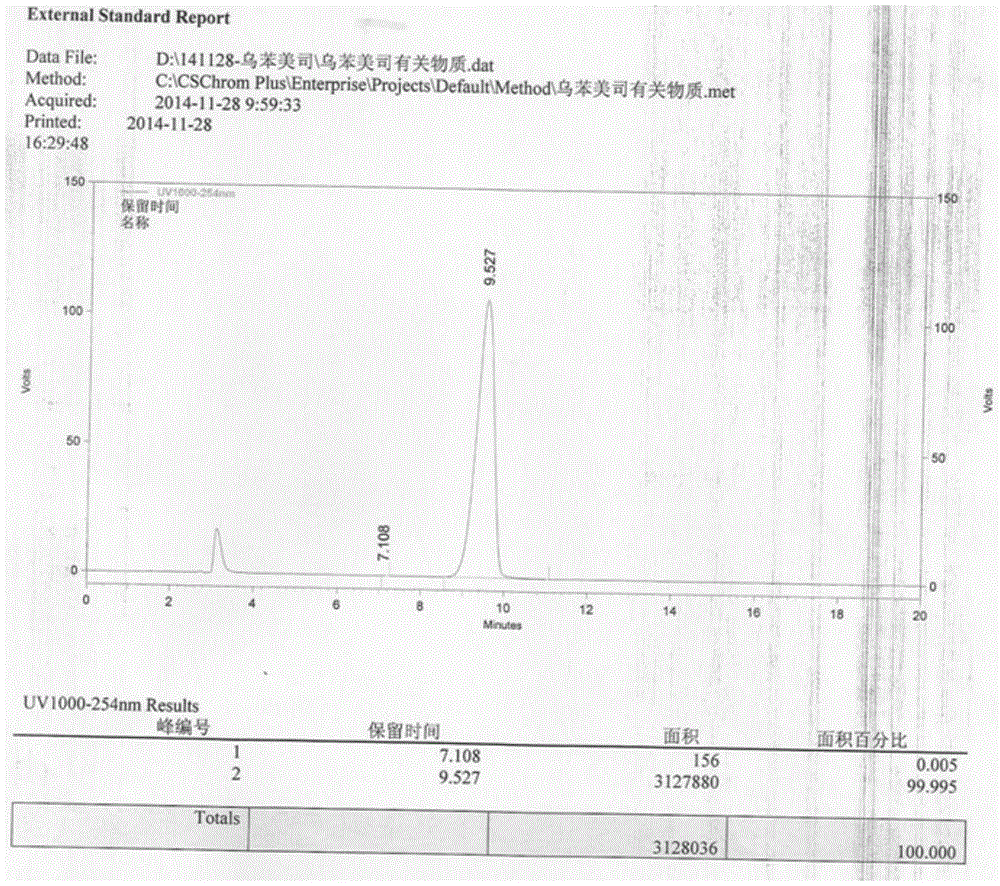
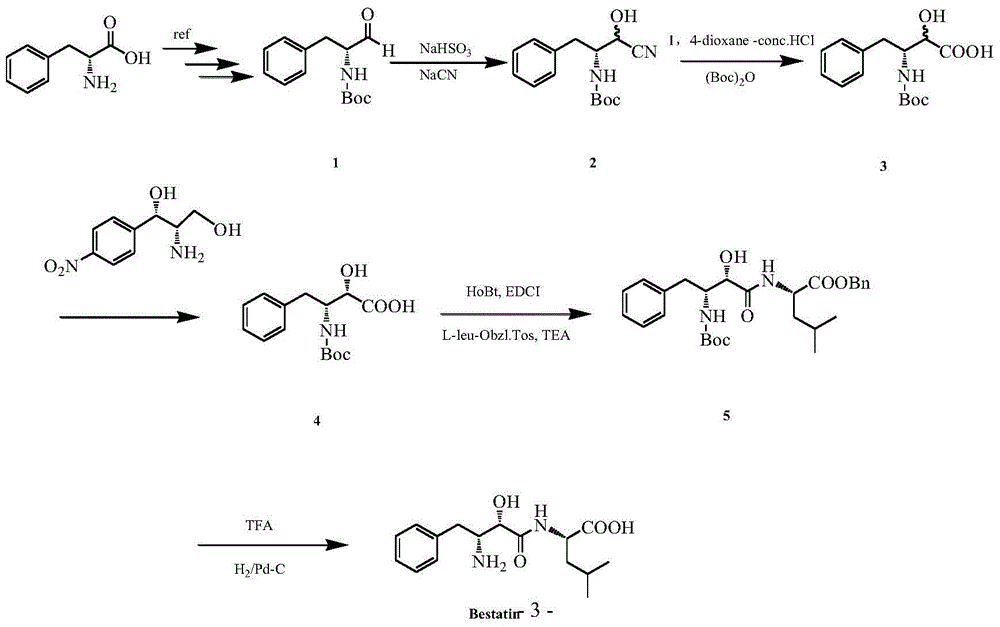
Image
Examples
Embodiment 1
[0025] Preparation of (2RS, 3R)-3-tert-butoxyamido-2-hydroxyl-4-phenylbutyronitrile (1):
[0026]Dissolve 36g (0.143mol) D-Boc-phenylalaninaldehyde in 100mL ethyl acetate, add 400mL distilled water, 17.6g (0.171mol) sodium bisulfite, monitor the reaction process by thin layer chromatography (TLC), and react after 10h Finish, separate and take water phase, wash 2 times with 300mL ethyl acetate, add 7.7g (0.157mol) sodium cyanide to water phase, react overnight at room temperature, after extracting 3 times with 300mL ethyl acetate, wash 3 times with 300mL water, organic The phase was dried over anhydrous sodium sulfate for 24 h, and evaporated to dryness to obtain 27 g of (2RS, 3R)-3-tert-butoxyamido-2-hydroxy-4-phenylbutyronitrile (1), with a yield of 68.5%.
[0027] Preparation of (2RS, 3R)-3-tert-butoxyamido-2-hydroxyl-4-phenylbutanoic acid (2):
[0028] Dissolve 25g (0.09mol) of (2RS, 3R)-3-tert-butoxyamido-2-hydroxy-4-phenylbutyronitrile (1) in 100mL of 1,4-dioxane, drop i...
Embodiment 2
[0036] Preparation of (2RS, 3R)-3-tert-butoxyamido-2-hydroxyl-4-phenylbutyronitrile (1):
[0037] Dissolve 21.6g (0.0858mol) D-Boc-phenylalaninaldehyde in 43mL ethyl acetate, add 173mL distilled water, 6.5g (0.103mol) sodium bisulfite, monitor the reaction process by thin layer chromatography (TLC), after 10h After the reaction was completed, the aqueous phase was separated, washed twice with 300 mL of ethyl acetate, added 2.16 g (0.094 mol) of sodium cyanide to the aqueous phase, reacted overnight at room temperature, extracted three times with 300 mL of ethyl acetate, washed three times with 300 mL of water, The organic phase was dried over anhydrous sodium sulfate for 24 h, and evaporated to dryness to obtain 15 g of (2RS, 3R)-3-tert-butoxyamido-2-hydroxy-4-phenylbutyronitrile (1), with a yield of 63.4%.
[0038] Preparation of (2RS, 3R)-3-tert-butoxyamido-2-hydroxyl-4-phenylbutanoic acid (2):
[0039] Dissolve 10g (0.036mol) of (2RS, 3R)-3-tert-butoxyamido-2-hydroxy-4-phe...
Embodiment 3
[0047] Preparation of (2RS, 3R)-3-tert-butoxyamido-2-hydroxyl-4-phenylbutyronitrile (1):
[0048] Dissolve 11g (0.043mol) of D-Boc-phenylalaninaldehyde in 33mL of ethyl acetate, add 132mL of distilled water, 8.8g (0.052mol) of sodium bisulfite, and monitor the reaction process by thin-layer chromatography (TLC). After 10 hours, the reaction End, separate the water phase, wash it twice with 300mL ethyl acetate, add 4.4g (0.048mol) sodium cyanide to the water phase, react at room temperature overnight, extract three times with 100mL ethyl acetate, wash three times with 100mL water, organic The phase was dried over anhydrous sodium sulfate for 24 h and evaporated to dryness to obtain 8 g of (2RS, 3R)-3-tert-butoxyamido-2-hydroxy-4-phenylbutyronitrile (1), with a yield of 66%.
[0049] Preparation of (2RS, 3R)-3-tert-butoxyamido-2-hydroxyl-4-phenylbutanoic acid (2):
[0050]Dissolve 17g (0.06mol) of (2RS, 3R)-3-tert-butoxyamido-2-hydroxy-4-phenylbutyronitrile (1) in 68mL of 1,4-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com