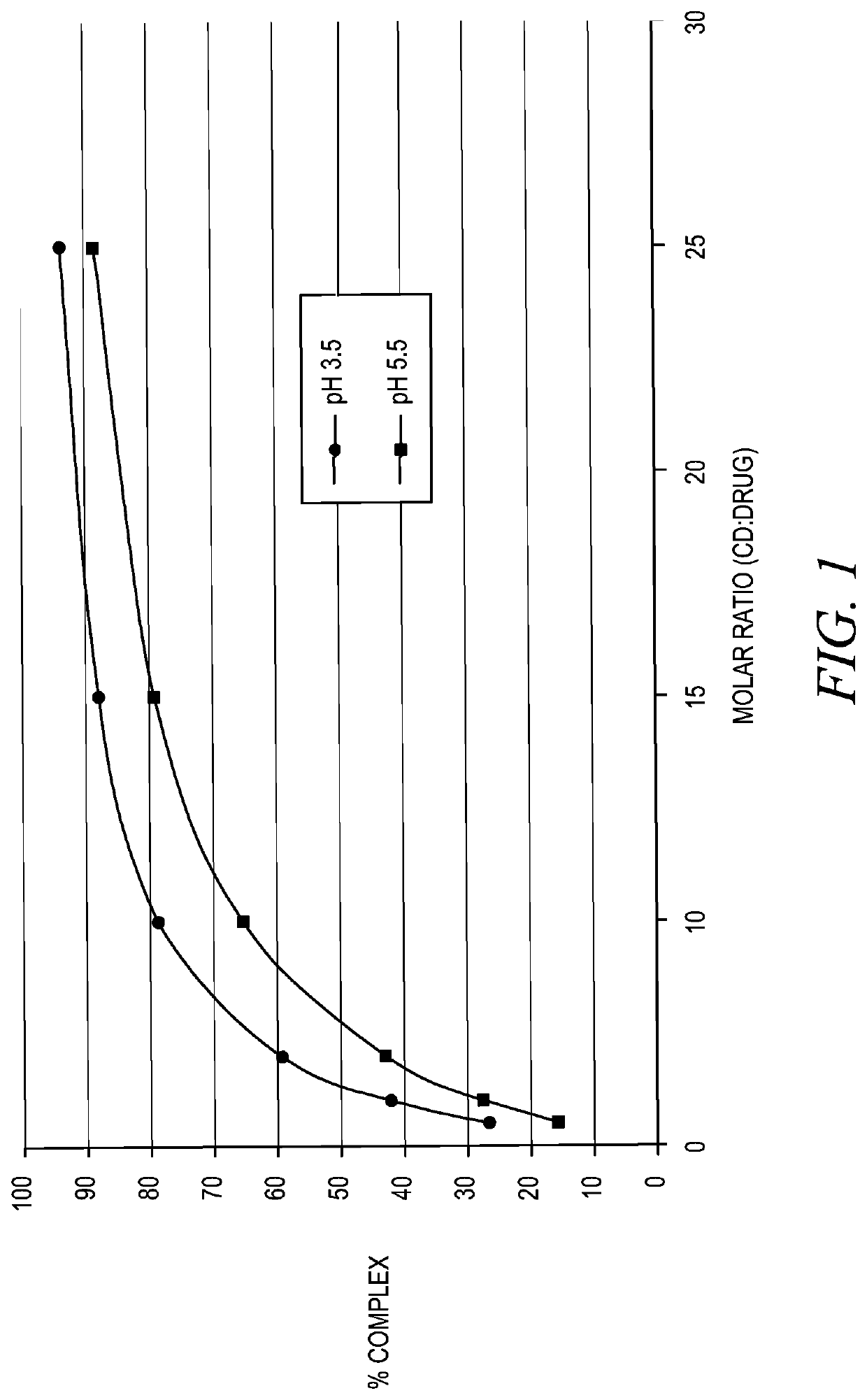
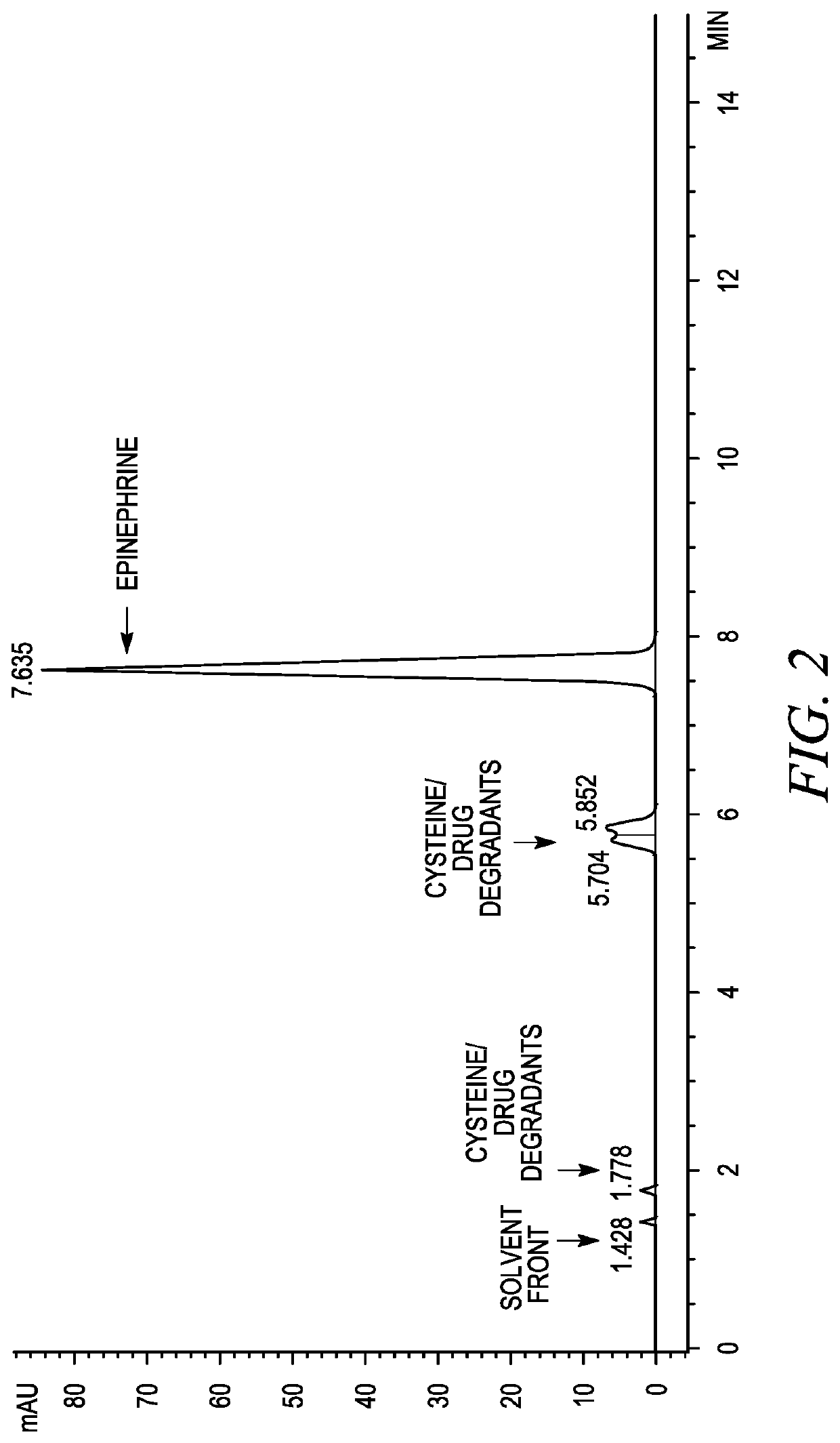
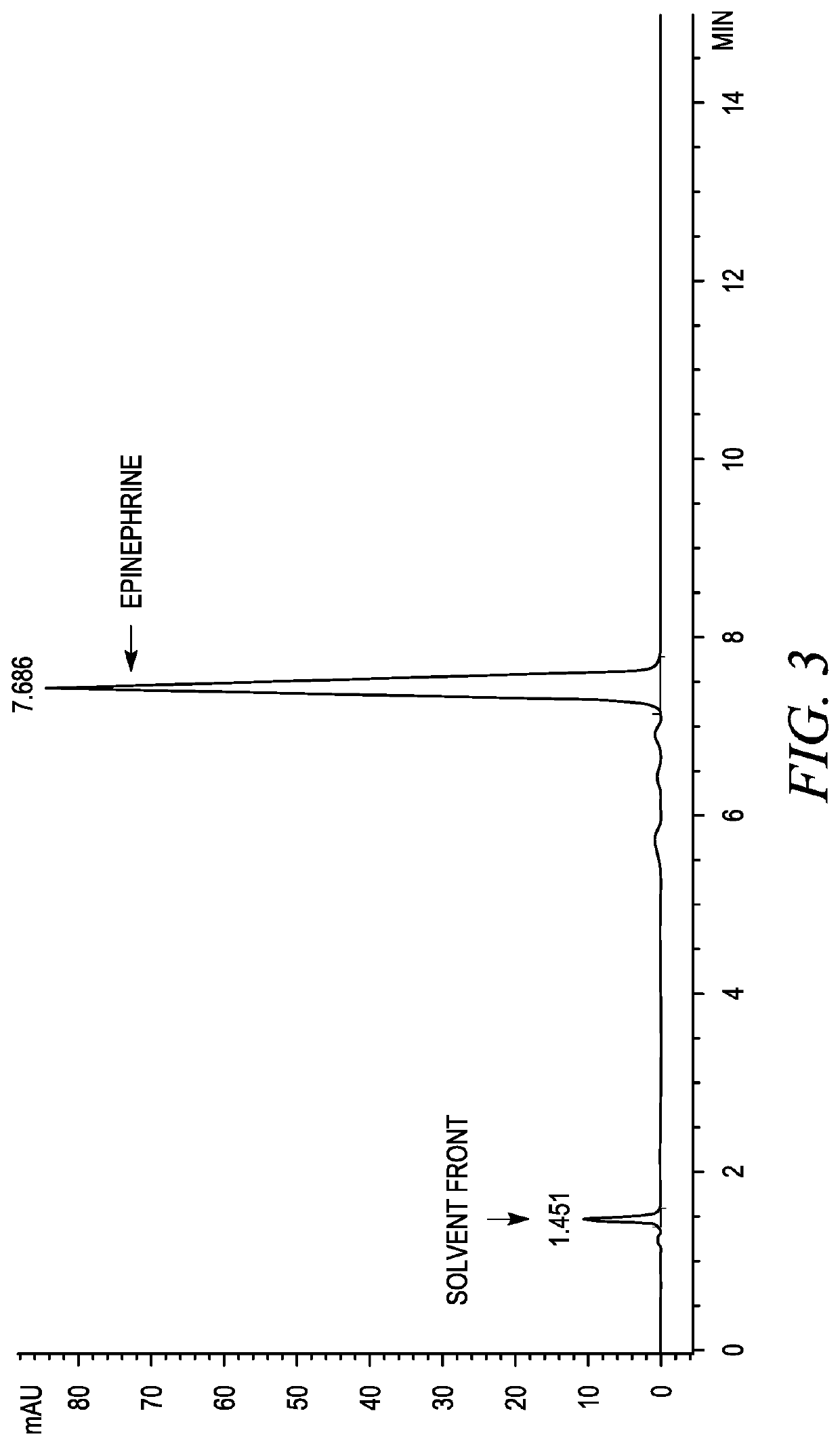
Stabilization of epinephrine formulations
a technology of epinephrine and stabilization, which is applied in the direction of pharmaceutical delivery mechanism, macromolecular non-active ingredients, organic active ingredients, etc., can solve the problems of inability to modify or degrade the catechol amine, the severity of the resulting anaphylactic reaction, and the sudden and severe systemic allergic reaction that can be fatal, etc., to achieve good physicochemical stability, improve physicochemical stability, and excellent physicochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0118]
IngredientConcentration (mg / mL)Epinephrine (as free base)1.0SBEβCD6.0Cysteine5.0Citric Acid5.0Edetate2.0Sodium Chloride7.8HCl and / or NaOH (pH 2.5 ± 0.1)q.s.Water for Injection (WFI)q.s.
example 2
[0119]
IngredientConcentration (mg / mL)Epinephrine (as free base)1.0SBEβCD6.0Cysteine5.0Citric Acid5.0Edetate2.0Sodium Chloride7.8HCl and / or NaOH (pH 3.5 ± 0.1)q.s.Water for Injection (WFI)q.s.
example 3
[0120]
IngredientConcentration (mg / mL)Epinephrine (as free base)1.0HPβCD4.0Cysteine5.0Citric Acid5.0Edetate2.0Sodium Chloride7.8HCl and / or NaOH (pH 2.5 ± 0.1)q.s.Water for Injection (WFI)q.s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com