Phenylephrine hydrochloride oral instant membrane and preparation method thereof
A technology of phenylephrine hydrochloride and oral instant film, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. The effect of high medication compliance and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
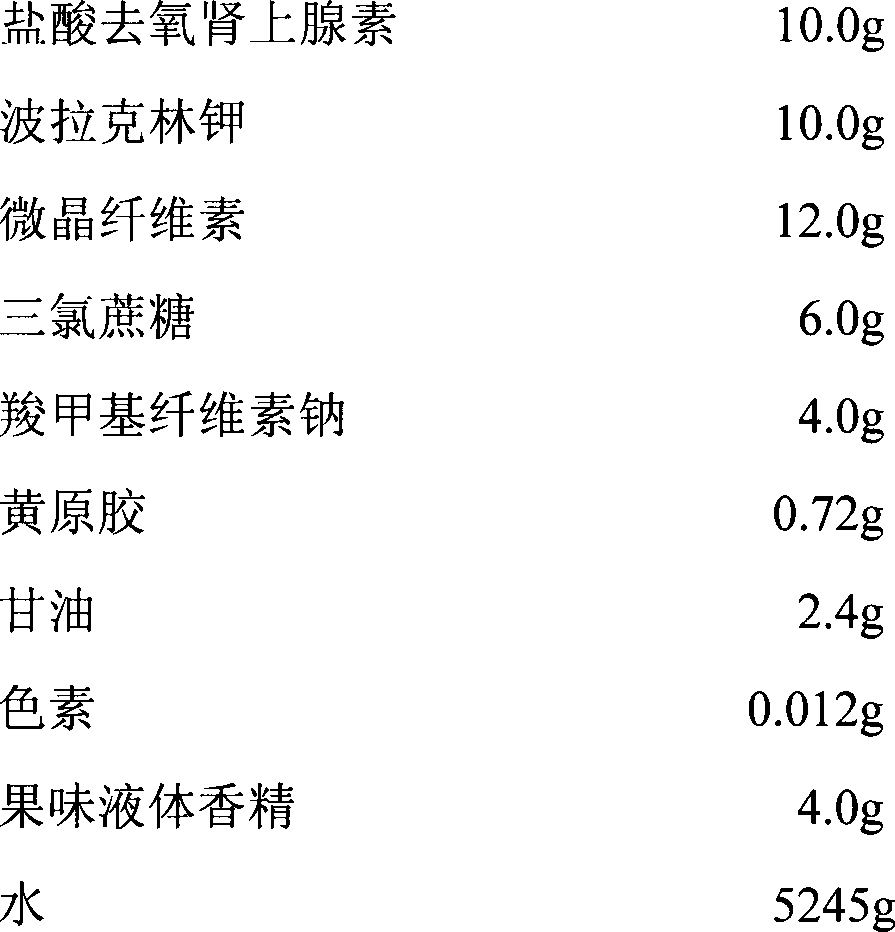
[0022] Each tablet contains 10.0mg of phenylephrine hydrochloride, and the composition of every 1000 tablets of phenylephrine hydrochloride film is:
[0023]
[0024] The preparation method of phenylephrine hydrochloride oral cavity instant film provided by the invention comprises the steps:
[0025] A. Prepare the drug resin suspension containing phenylephrine hydrochloride: take phenylephrine hydrochloride, dissolve it with 500 times (V / W) water, add polacrilin potassium resin after stirring and dissolving at room temperature, and continue stirring Ion exchange for 1 hour to obtain phenylephrine hydrochloride drug resin suspension;
[0026] B. Mixing and film coating: Add microcrystalline cellulose and sucralose to the suspension obtained in A, stir to disperse evenly, then add sodium carboxymethylcellulose, let it stand for swelling for 1 hour, and then add it under stirring 0.3% xanthan gum aqueous solution, after stirring evenly, add 30% glycerin aqueous solution, 0.3...
Embodiment 2
[0030] Each tablet contains 10.0mg of phenylephrine hydrochloride, and the composition of every 1000 tablets of phenylephrine hydrochloride film is:
[0031]
[0032] The preparation method of phenylephrine hydrochloride oral cavity instant film provided by the invention comprises the steps:
[0033] A. Prepare the drug resin suspension containing phenylephrine hydrochloride: take phenylephrine hydrochloride, dissolve it with 500 times (V / W) water, add polacrilin potassium resin after stirring and dissolving at room temperature, and continue stirring Ion exchange for 1 hour to obtain phenylephrine hydrochloride drug resin suspension;
[0034] B. Mixing and film coating: add microcrystalline cellulose and aspartame to the suspension obtained in A, stir to disperse evenly, then add hypromellose, let it stand for swelling for 1 hour, and then add it under stirring 0.3% xanthan gum aqueous solution, after stirring evenly, add 30% glycerin aqueous solution, 0.3% carmine pigment...
Embodiment 3
[0038] Each tablet contains 10.0mg of phenylephrine hydrochloride, and the composition of every 1000 tablets of phenylephrine hydrochloride film is:
[0039]
[0040]
[0041] The preparation method of phenylephrine hydrochloride oral cavity instant film provided by the invention comprises the steps:
[0042] A. Preparation of drug resin suspension containing phenylephrine hydrochloride: take phenylephrine hydrochloride, dissolve it in 500 times (V / W) water, add polacrilin bell resin after stirring and dissolving at room temperature, and continue stirring Ion exchange for 1 hour to obtain phenylephrine hydrochloride drug resin suspension;
[0043] B. Mixing and film coating: Add microcrystalline cellulose and sucralose to the suspension obtained in A, stir to disperse evenly, then add sodium carboxymethylcellulose, let it stand for swelling for 1 hour, and then add it under stirring 0.3% xanthan gum aqueous solution, after stirring evenly, add 30% glycerin aqueous solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com