Compound pharmaceutical composition for treating rhinitis in children
A composition and compound technology, applied in the field of pharmacy, can solve the problems of undiscovered pharmaceutical composition patent applications or reports, and achieve the effects of short time-consuming, low cost, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
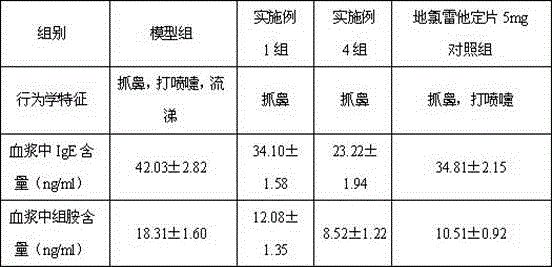
Examples
Embodiment 1
[0049] The prescription amount (1000 bags) and the preparation method of the compound pharmaceutical composition for rhinitis in children made into granules according to the present invention are as follows:
[0050] Element
Amount per 1000 bags (g)
2
3 --> Phenylephrine Hydrochloride
4
11
Hydroxypropyl-β-cyclodextrin
8
500
100
300
fragrance
10
Appropriate amount
[0051] Preparation Process:
[0052] (1) Weigh the raw and auxiliary materials according to the prescription amount, pass through a 100-mesh sieve for later use;
[0053] (2) Add chlorpheniramine maleate and phenylephrine hydrochloride to the hydroxypropyl-β-cyclodextrin solution, stir well and then add sucrose to make mixture 1;
[0054] (3) Take dipotassium glycyrrhizinate and mix it with t...
Embodiment 2
[0056] The prescription quantity (1000 tablets) and the preparation method of the compound pharmaceutical composition for rhinitis in children made into orally disintegrating tablets according to the present invention are as follows:
[0057] Element
Dosage per 1000 tablets (g)
2
4
11
Polacrilin Potassium Resin (IRP88)
6
100
50
Polyacrylic resin IV
15
Low-substituted hydroxypropyl cellulose
15
8
2
fragrance
10
75% ethanol solution
Appropriate amount
Appropriate amount
[0058] Preparation Process:
[0059] (1) Weigh the raw and auxiliary materials according to the prescription quantity;
[0060] (2) Prepare 5% polyacrylic acid resin IV ethanol solution as ...
Embodiment 3
[0065] The prescription amount (1000 tablets) and the preparation method of the compound pharmaceutical composition for children's rhinitis made into oral dissolving film according to the present invention are as follows:
[0066] Element
Dosage per 1000 tablets (g)
2
4
11
Polacrilin Potassium Resin (IRP88)
10
Methylcellulose
12
4
4
Glycerin
5
1
2
Carmine
0.02
Appropriate amount
[0067] Preparation Process:
[0068] (1) Weigh the raw and auxiliary materials according to the prescription quantity;
[0069] (2) Dissolve chlorpheniramine maleate and phenylephrine hydrochloride in an appropriate amount of purified water, add polacrilin potassium resin and stir ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com