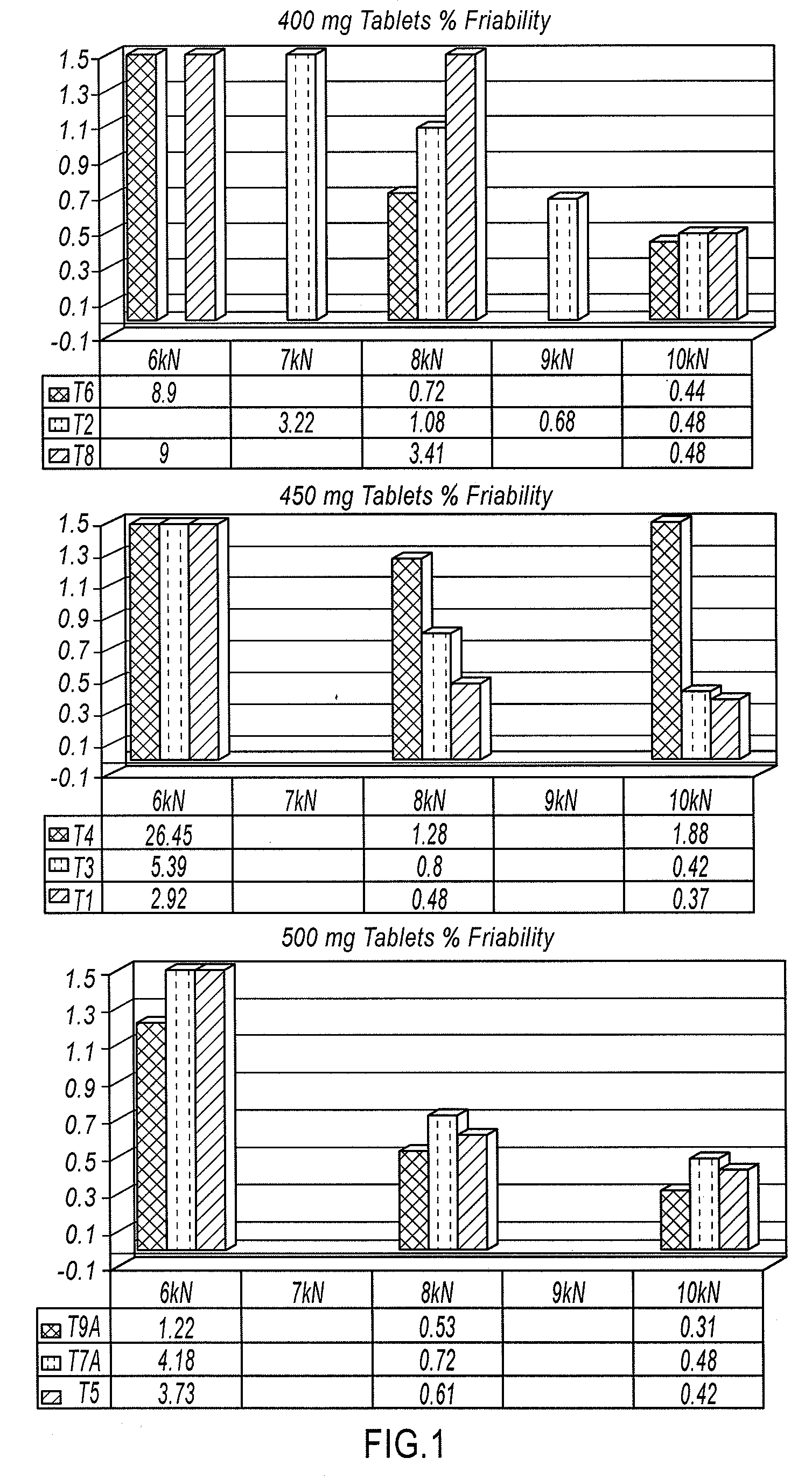
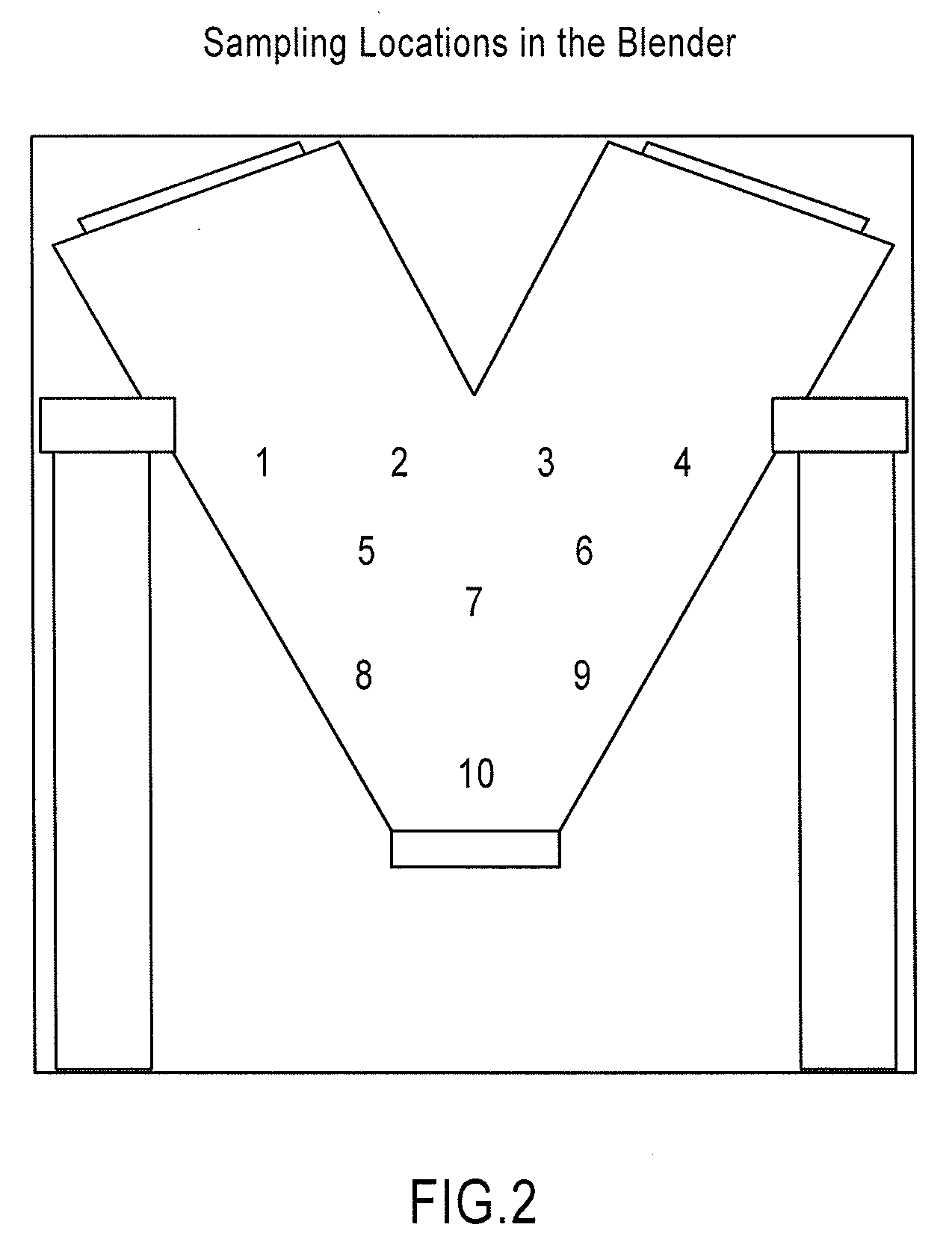
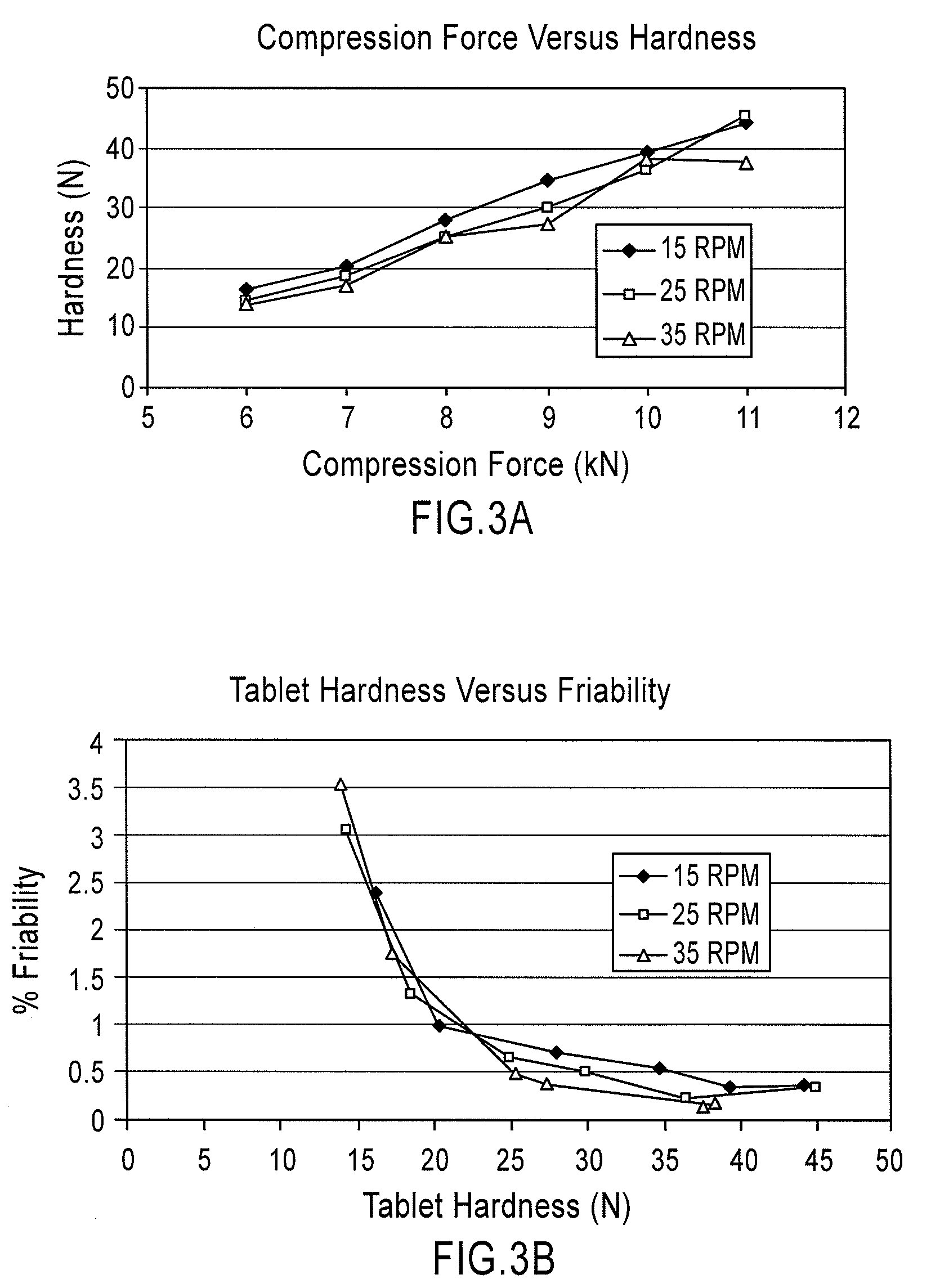
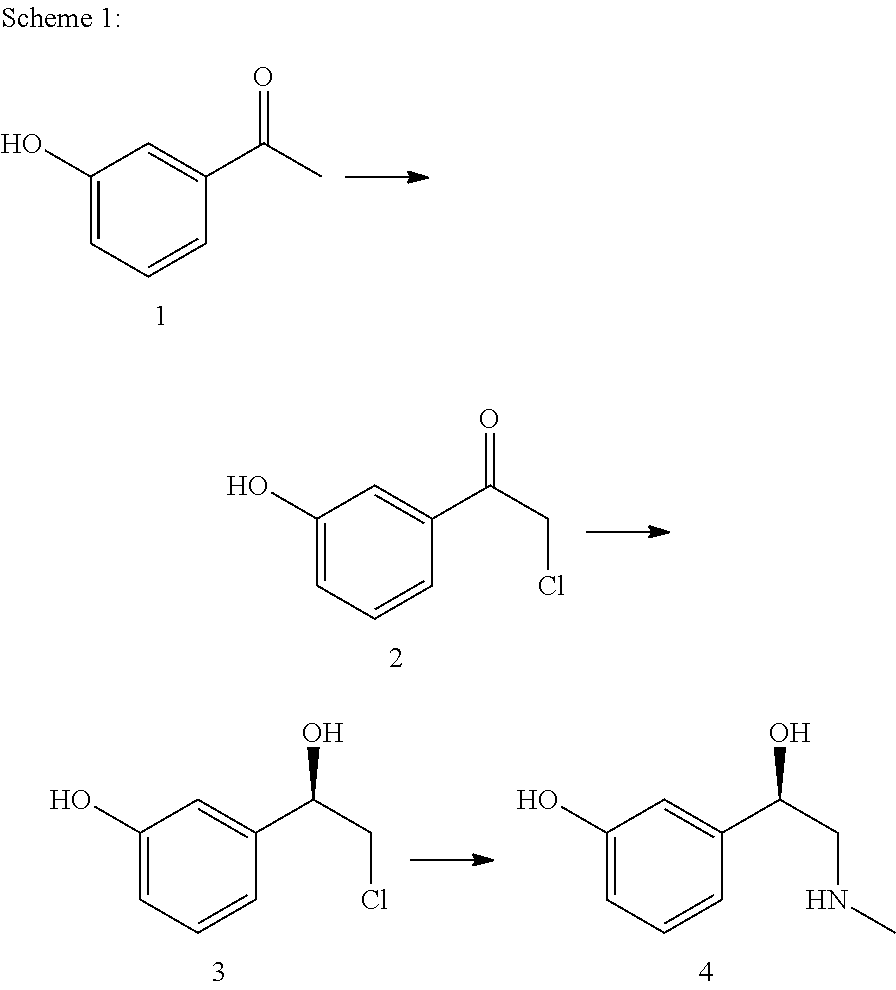
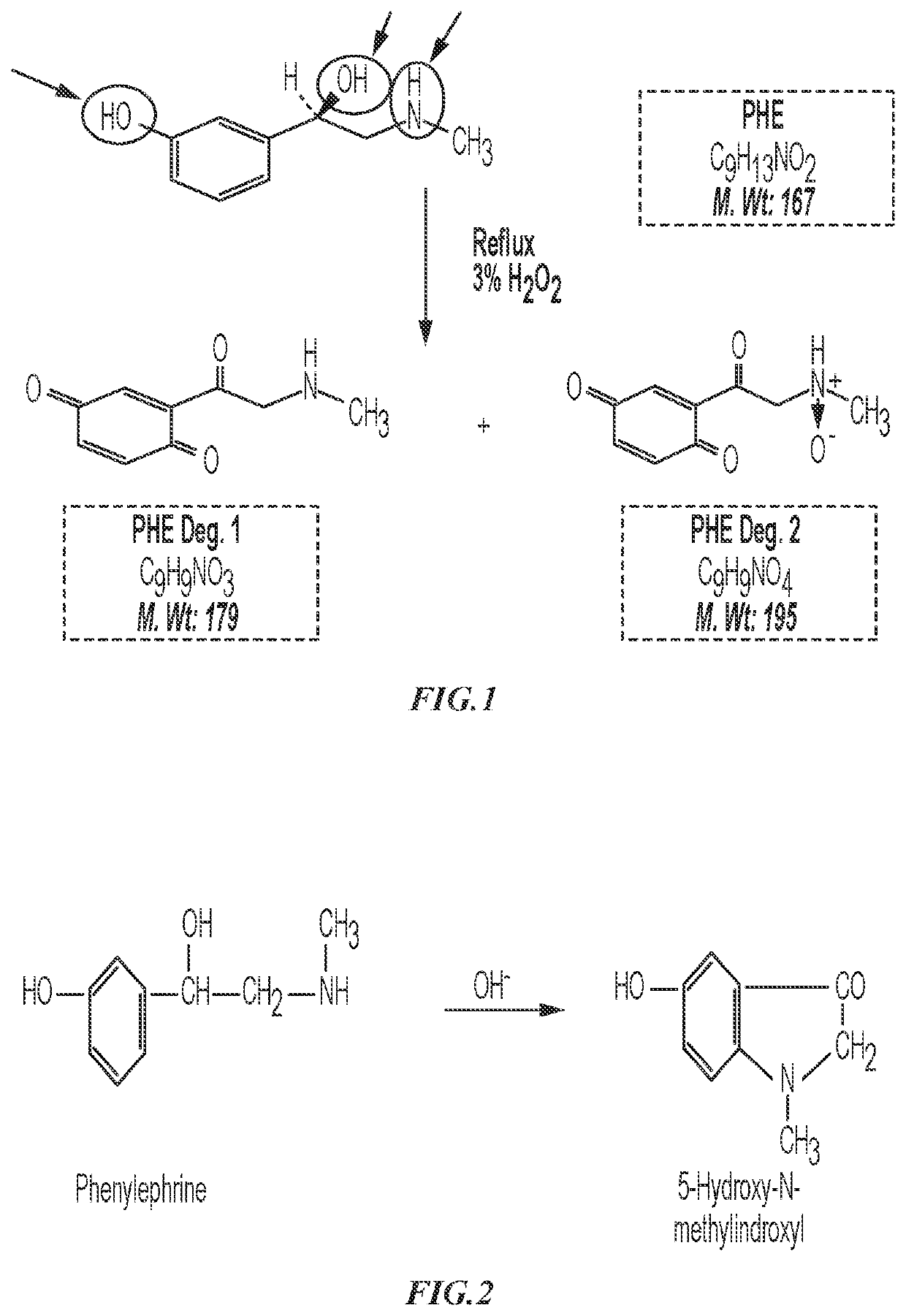
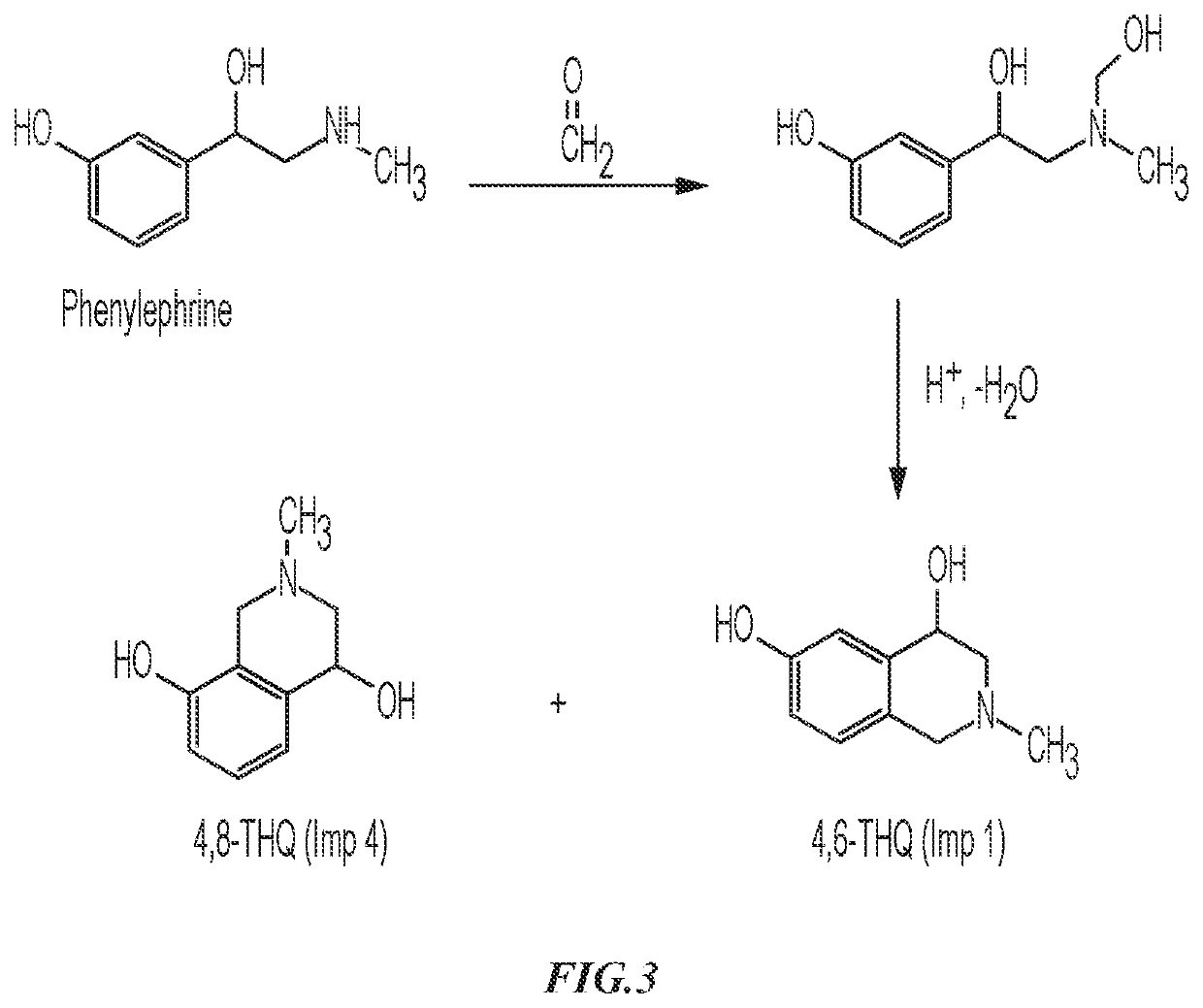
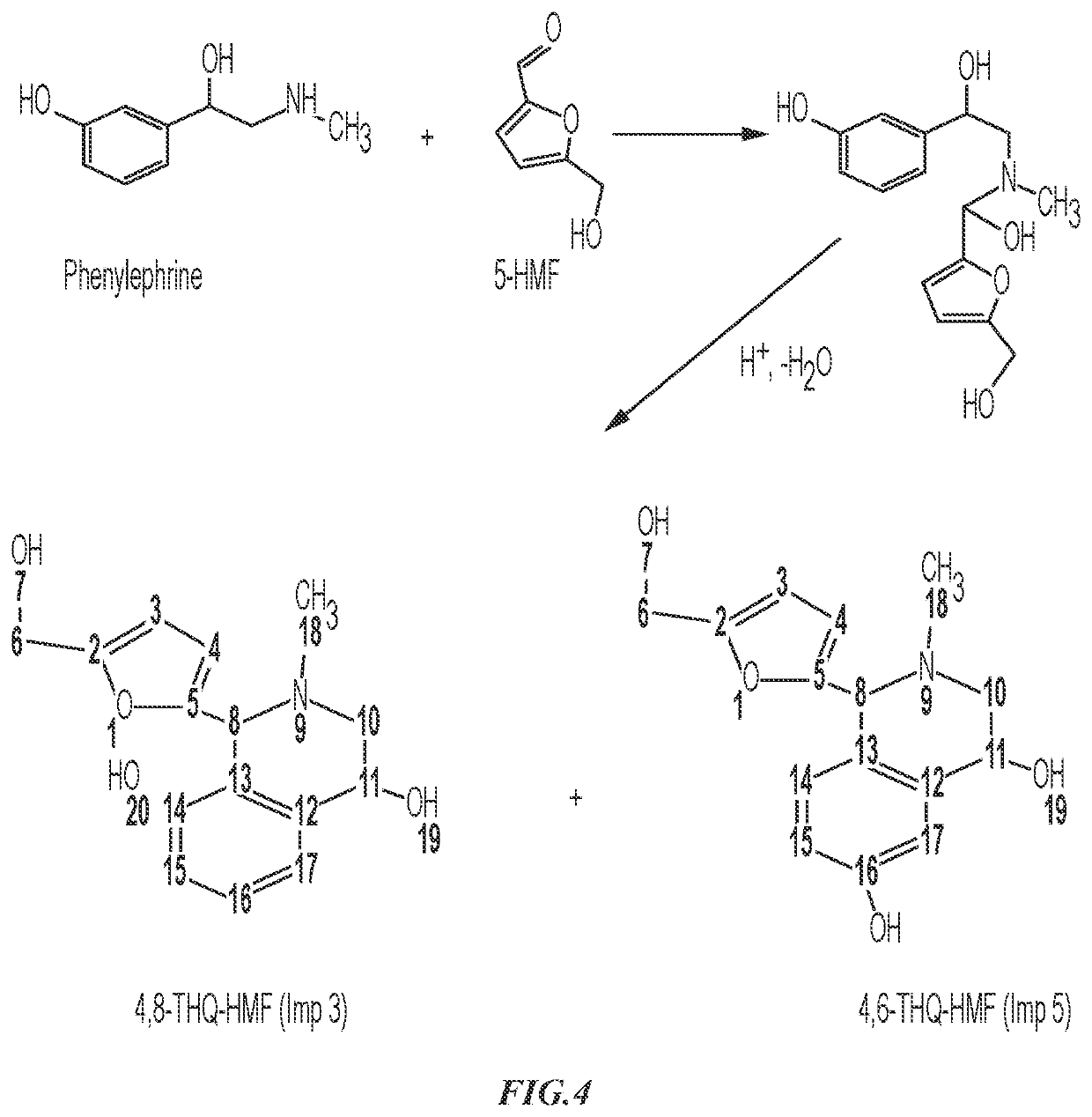
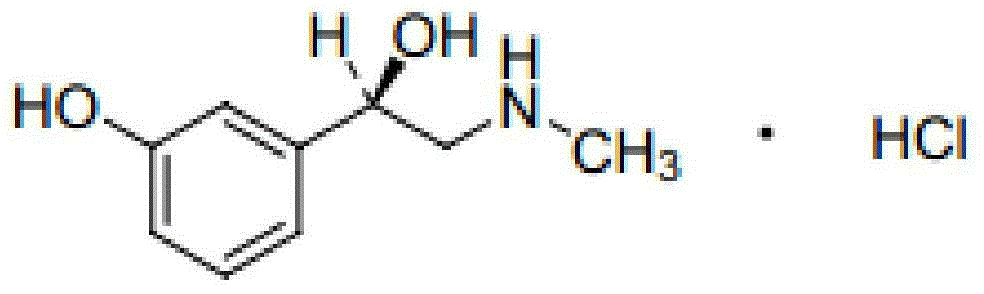Patents
Literature
49 results about "Norphenylephrine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
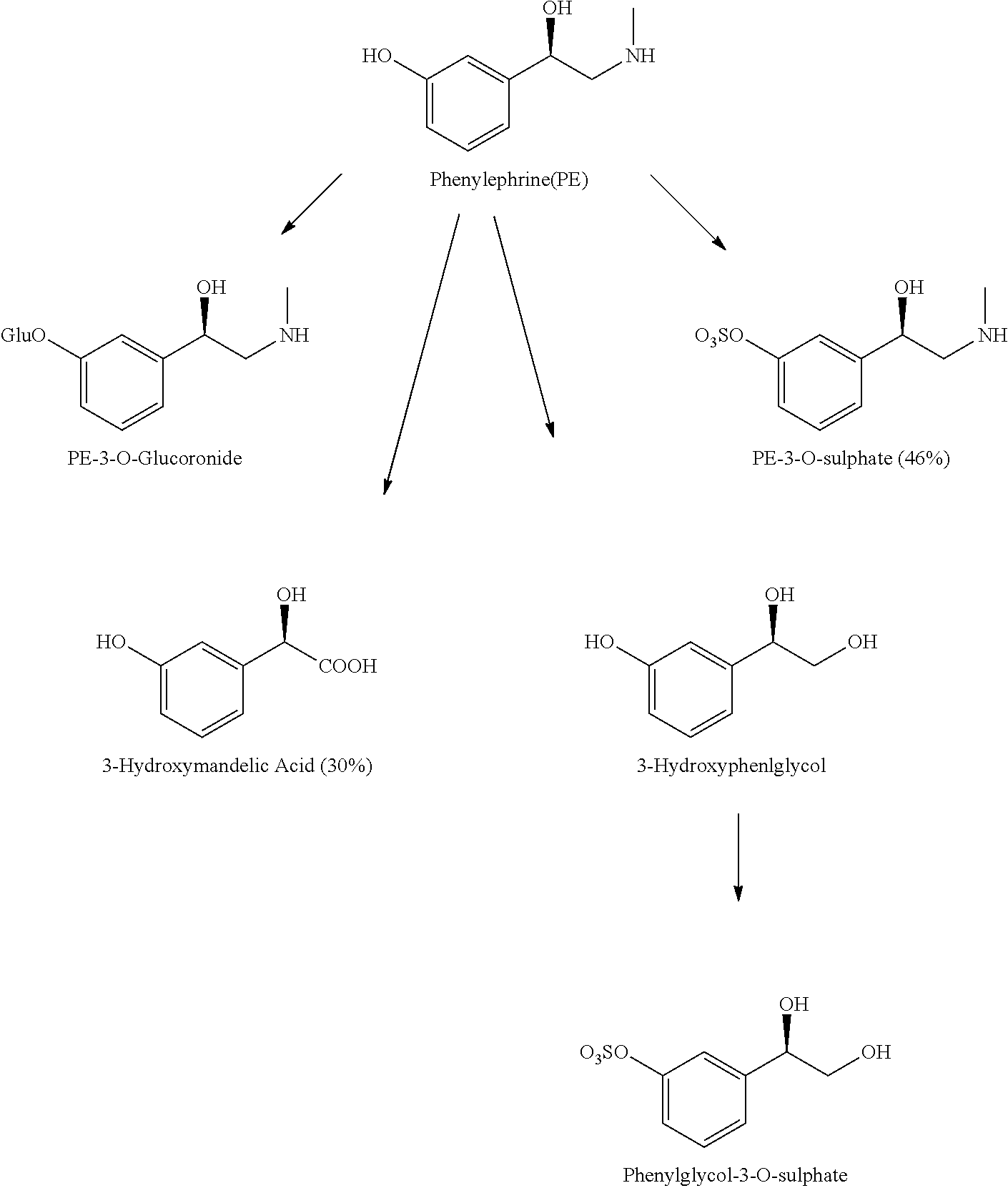
Norfenefrine or meta-octopamine (3-octopamine), also known as 3,β-dihydroxyphenethylamine, is an adrenergic agent used as a sympathomimetic drug which is marketed in Europe, Japan, and Mexico. ... Norphenylephrine is the precursor used to make Ciclafrine. References This drug article ...
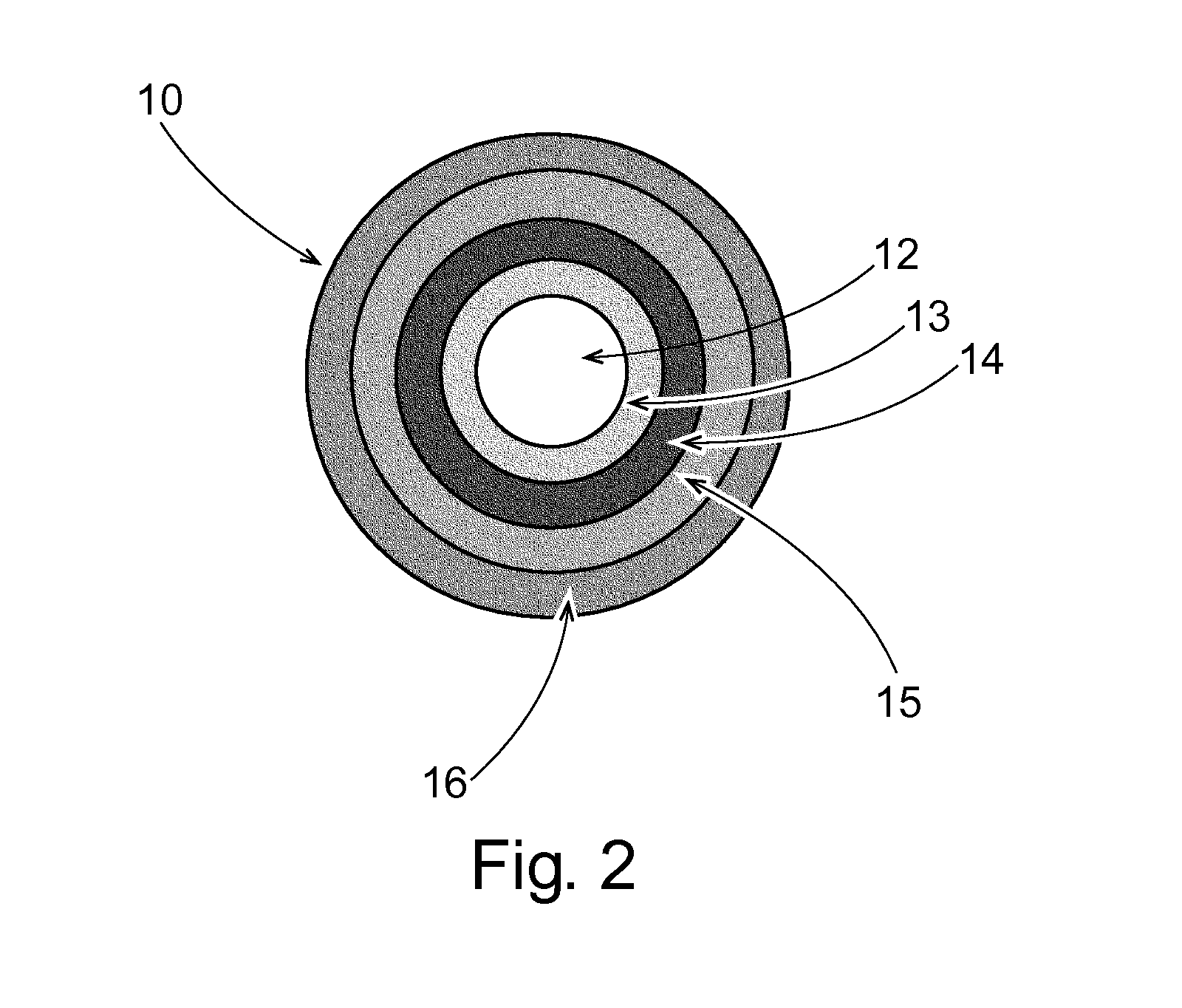
Phenylepherine containing dosage form
ActiveUS20060057205A1Good treatment effectRelieve symptomsBiocidePill deliveryNorphenylephrineBlood plasma
A pharmaceutical dosage form which comprises phenylepherine or a pharmaceutically acceptable salt thereof and a second drug. The dosage form provides a plasma concentration within the therapeutic range of the second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of phenylepherine. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:CAPELLON PHARMA LLC
Compositions and methods useful for treatment of respiratory illness
Disclosed are compositions including phenylephrine, its free and addition salt forms, and mixtures thereof, alone, or in combination with other pharmaceutical actives. The compositions have a pH of about 2 to about 5 and are substantially free of aldehydes. Also disclosed are methods of treating respiratory illness through administration of a composition comprising phenylephrine, its free and addition salt forms, and mixtures thereof alone, or in combination with other pharmaceutical actives, wherein the composition has a pH of from about 2 to about 5 and is substantially free of aldehydes.
Owner:THE PROCTER & GAMBLE COMPANY
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions comprising an antitussive, a decongestant and an expectorant, and in a specific embodiment comprising hydrocodone, phenylephrine hydrochloride and guaifenesin, wherein the composition may be substantially free of added sugar and added alcohol, and methods for using these compositions for the treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
Compositions and methods useful for treatment of respiratory illness
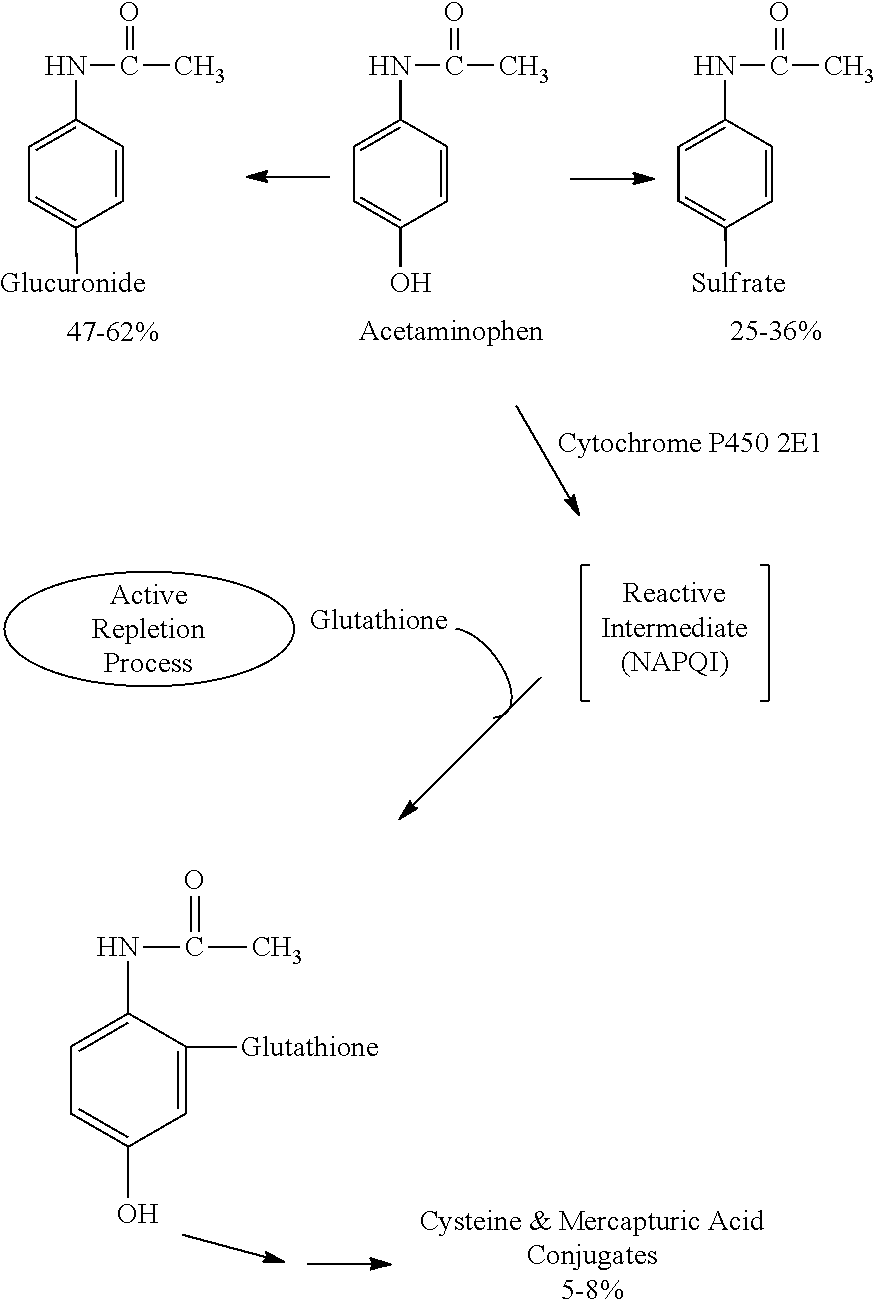
Disclosed are compositions including phenylephrine, its salts, and mixtures thereof, in combination with acetaminophen; and optionally in combination with additional pharmaceutical actives. The compositions have a pH of about 6.5 to about 7.5 and may be substantially free of aldehydes. The invention also provides a method of stabilizing phenylephrine. Also disclosed are methods of treating respiratory illness through administration of a composition comprising phenylephrine, its salts, and mixtures thereof, in combination with acteaminophen; and optionally in combination with additional pharmaceutical actives, wherein the composition has a pH of from about 6.5 to about 7.5 and may be substantially free of aldehydes.
Owner:THE PROCTER & GAMBLE COMPANY
Method for producing l-phenylephrine using an alcohol dehydrogenase of aromatoleum aromaticum ebn1 (azoarcus sp. ebn1)
ActiveUS20110171700A1High stereoselectivityMoreOrganic compound preparationCarbonyl compound preparationAlcoholAzoarcus sp.
The present invention relates to a multi-stage process for producing substituted, optically active alcohols, comprising an enzyme-catalyzed synthesis step, in particular a synthesis step which is catalyzed by an alcohol dehydrogenase. The inventive method is particularly suitable for producing phenylephrine, i.e. 3-[(1R)-1-hydroxy-2-methylamino-ethyl]-phenol.
Owner:BASF AG
Therapeutic formulations for the treatment of cold and flu-like symptoms
A pharmaceutical formulation of therapeutically effective amounts of acetaminophen, ibuprofen, and a sympathomimetic drug, such as pseudoephedrine (or its prodrug), or phenylephrine used in the treatment of cold and flu-like symptoms. Such symptoms may include fever, pain, nasal congestion, sinus congestion, runny nose, sore throat, myalgia, ear pressure and fullness, and headache. The formulation further includes various excipients used in the formulation process.
Owner:KINGSWAY PHARMA
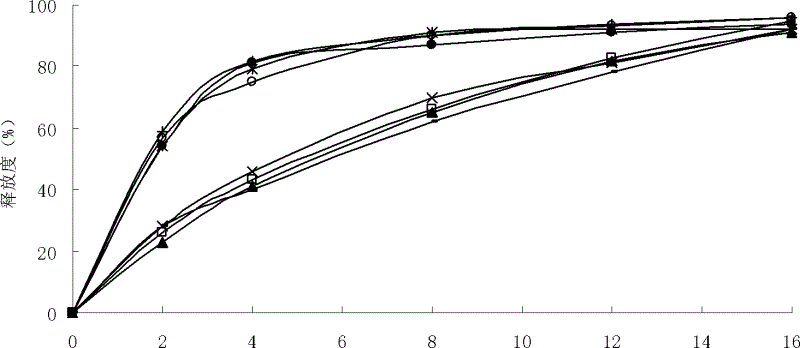
Phenylephrine pulsed release formulations and pharmaceutical compositions
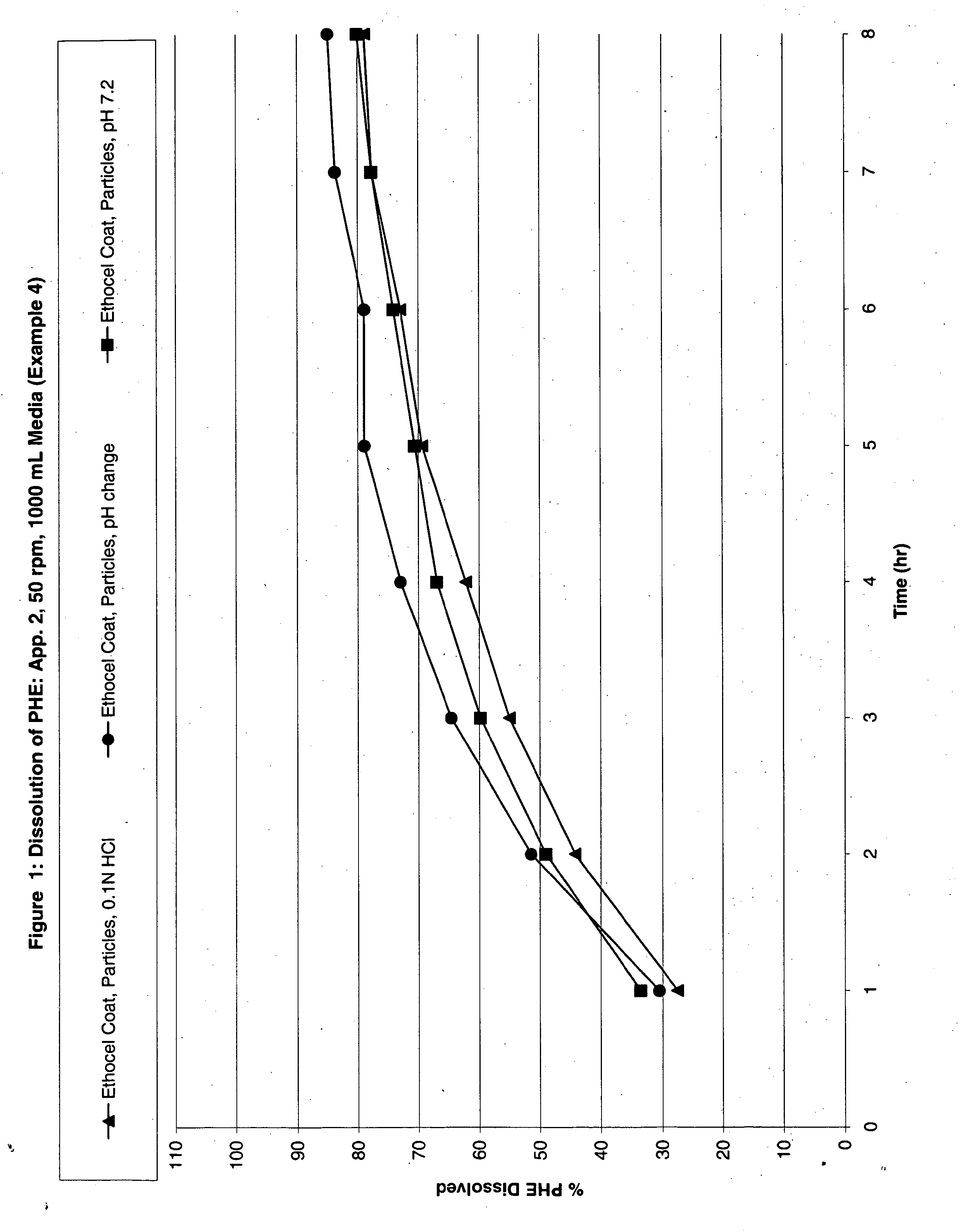
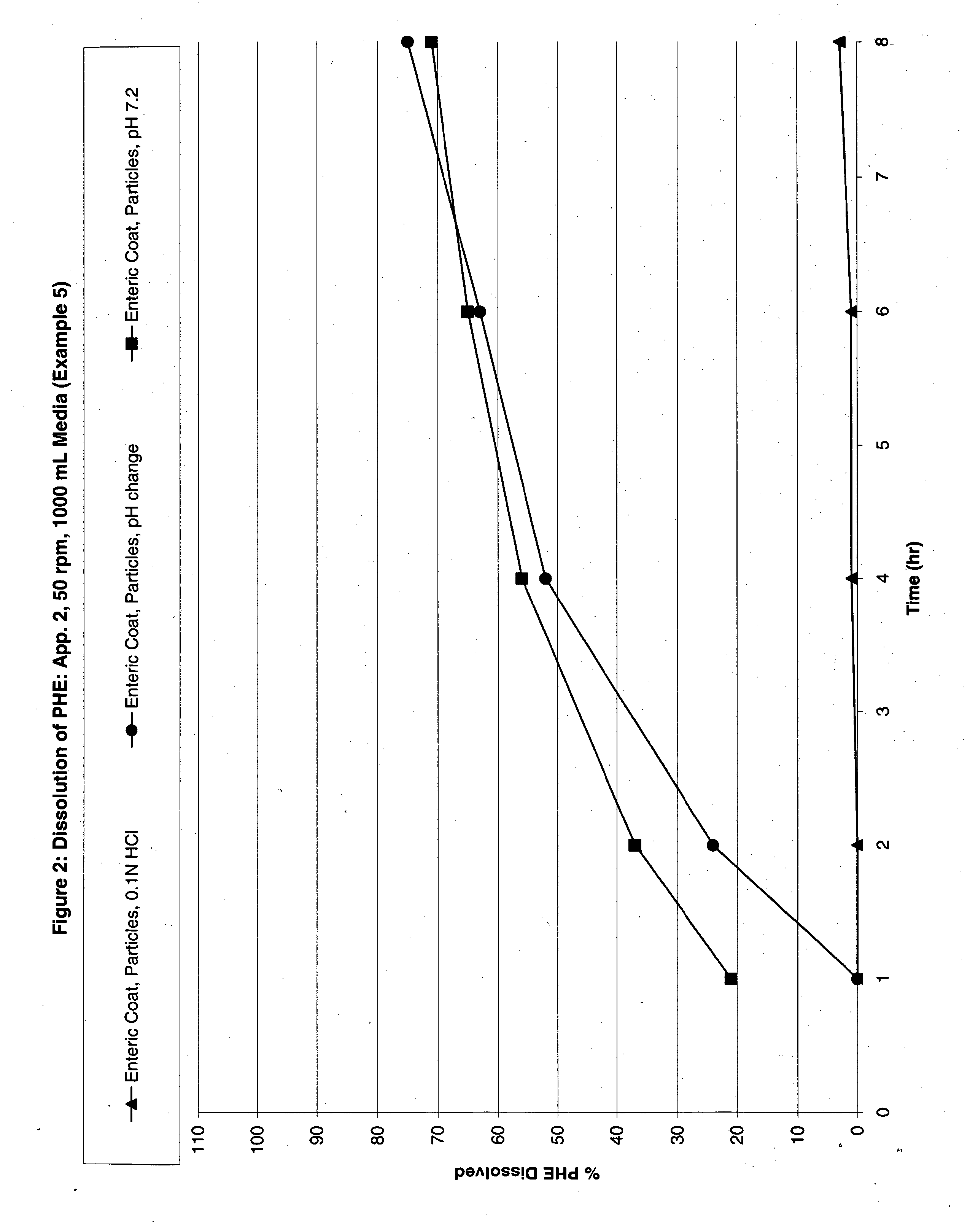
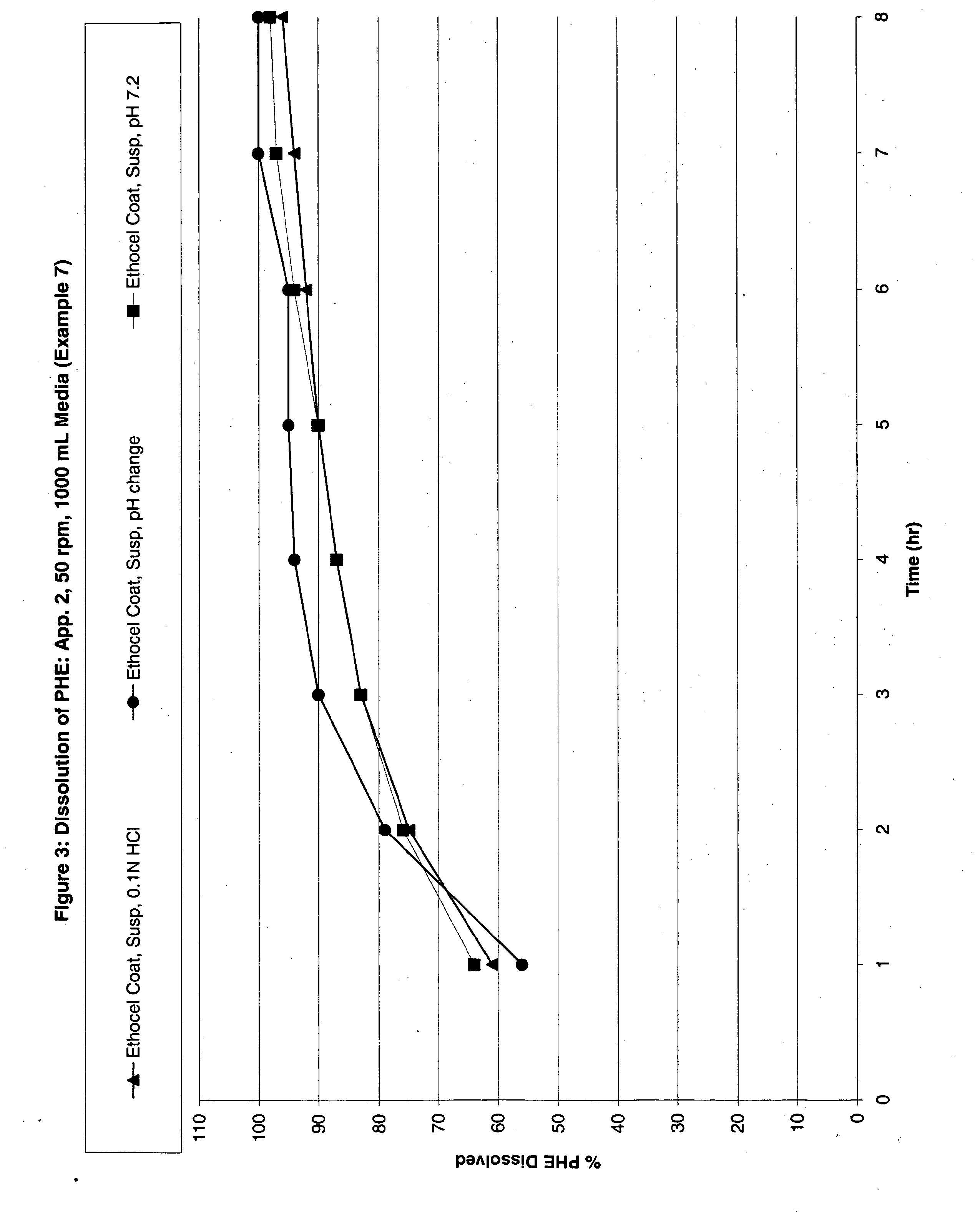
The invention discloses a pulsed-release formulation or a pharmaceutical composition comprising phenylephrine. The pharmaceutical composition comprises an immediate-release component and an enteric-coated component formulated together either in solid form or in a suspension. The enteric-coated component comprises microcrystals seeded with phenylephrine as an active ingredient and coated with a pH sensitive coating to delay release of the phenylephrine. The pharmaceutical composition can further comprise at least one active selected from the group consisting of an antihistamine, an analgesic, anti-pyretic, non-steroidal anti-inflammatory and mixtures of two or more said actives.
Owner:SCHERING CORP
Method for producing L-phenylephrine using an alcohol dehydrogenase of Aromatoleum aromaticum EBN1 (Azoarcus sp. EBN1)
ActiveUS8617854B2MoreHigh stereoselectivityOrganic compound preparationCarbonyl compound preparationAlcoholAzoarcus sp.
The present invention relates to a multi-stage process for producing substituted, optically active alcohols, comprising an enzyme-catalyzed synthesis step, in particular a synthesis step which is catalyzed by an alcohol dehydrogenase. The inventive method is particularly suitable for producing phenylephrine, i.e. 3-[(1R)-1-hydroxy-2-methylamino-ethyl]-phenol.
Owner:BASF AG
Compositions and methods useful for treatment of respiratory illness
Disclosed are compositions including phenylephrine, its free and addition salt forms, and mixtures thereof, alone, or in combination with other pharmaceutical actives. The compositions have a pH of about 2 to about 5 and are substantially free of aldehydes. Also disclosed are methods of treating respiratory illness through administration of a composition comprising phenylephrine, its free and addition salt forms, and mixtures thereof alone, or in combination with other pharmaceutical actives, wherein the composition has a pH of from about 2 to about 5 and is substantially free of aldehydes.
Owner:PROCTER & GAMBLE CO
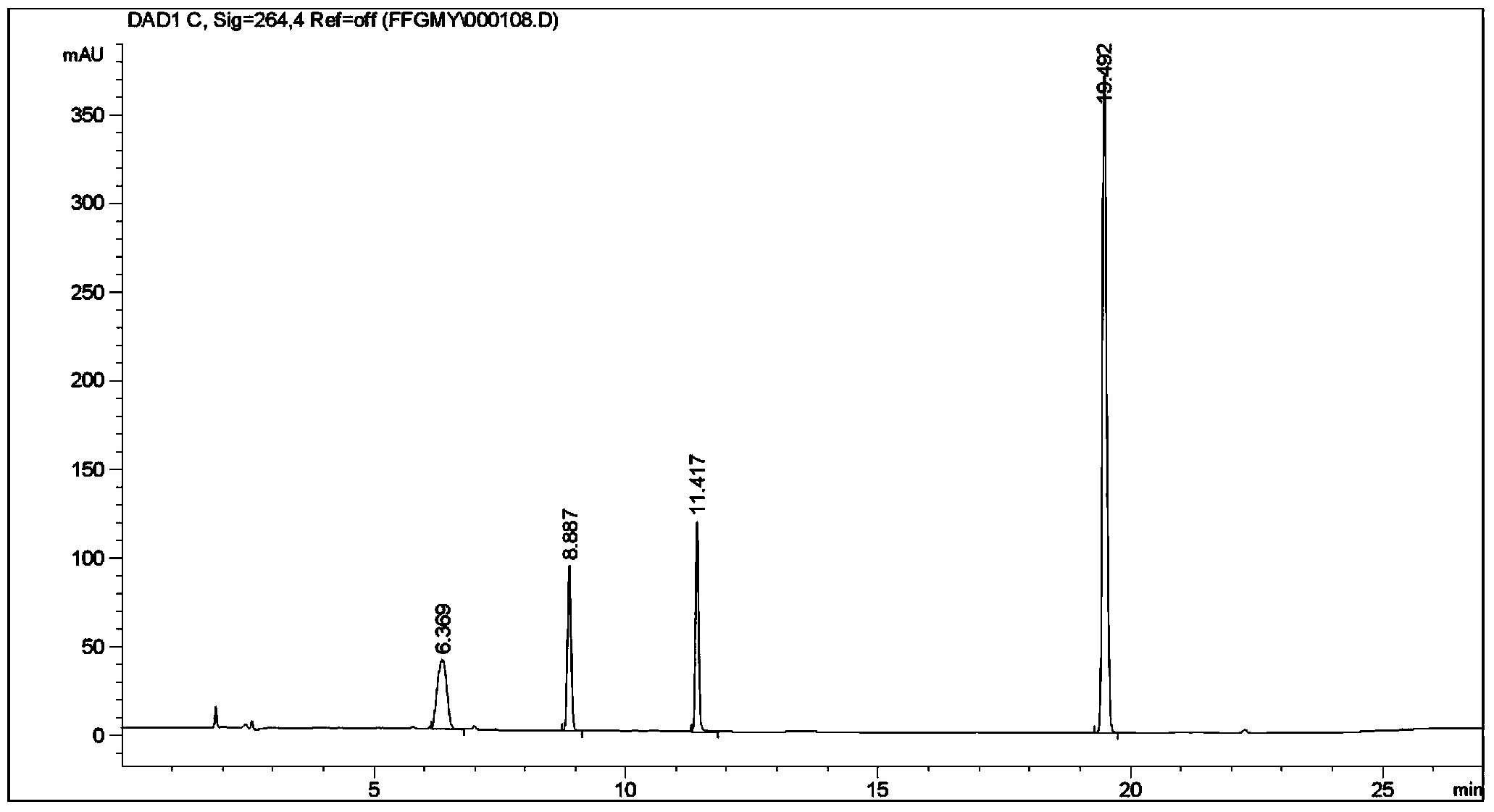
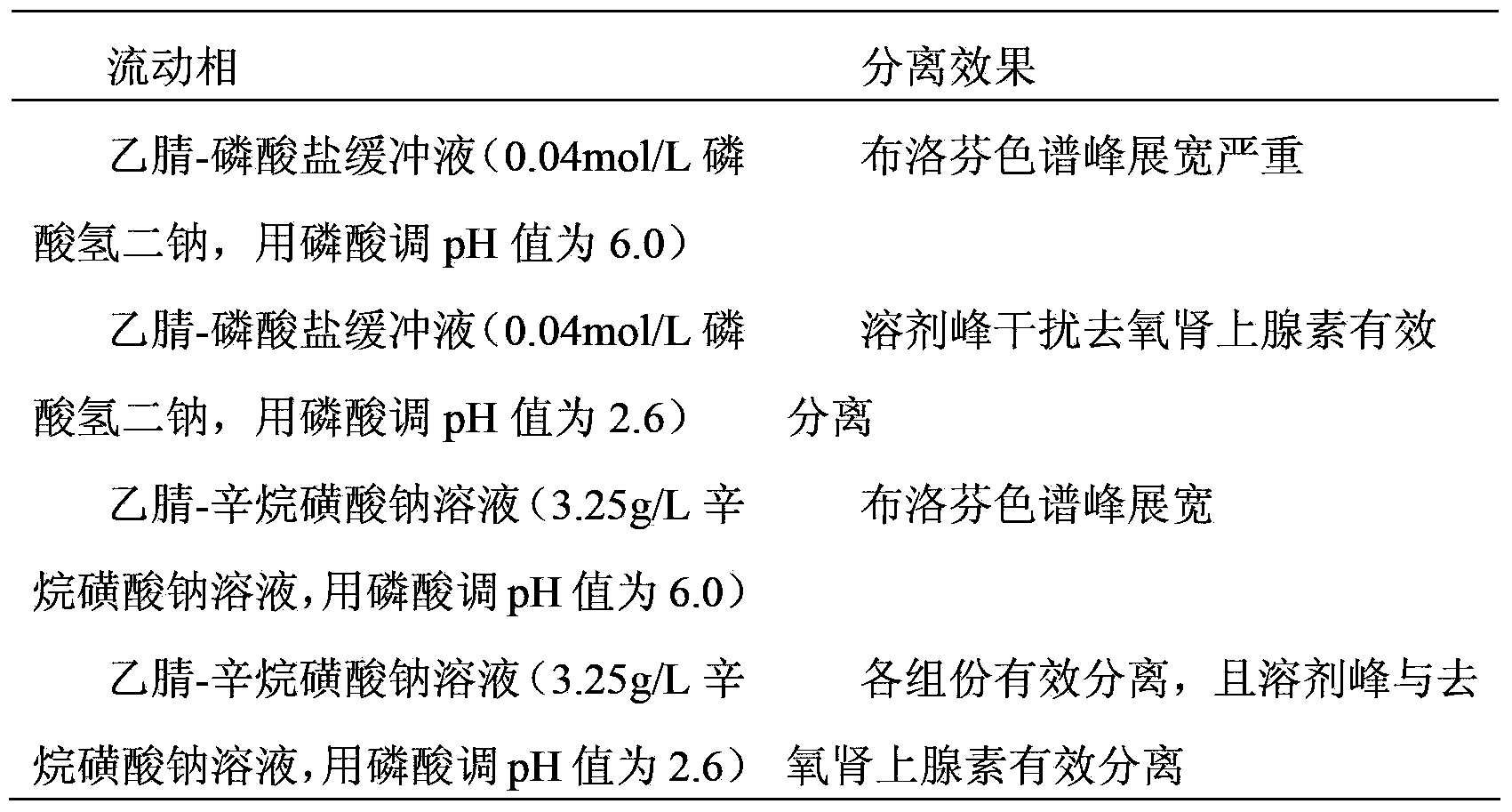
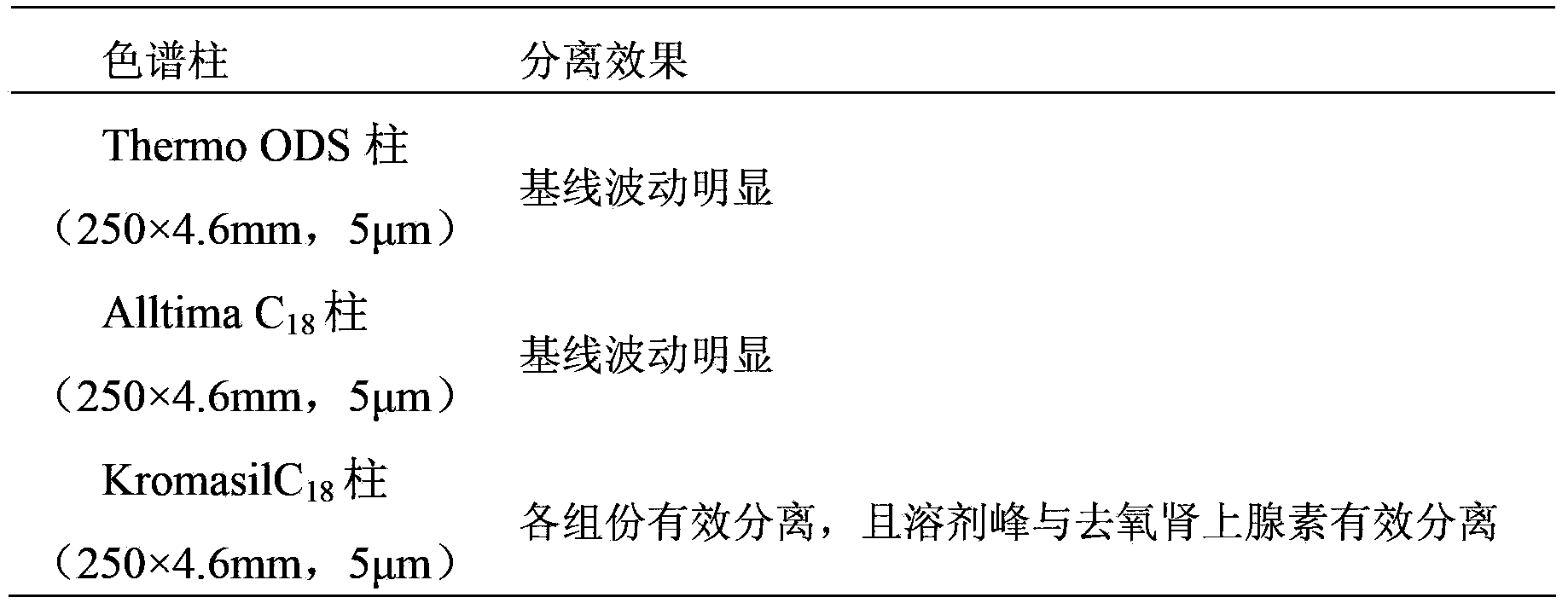
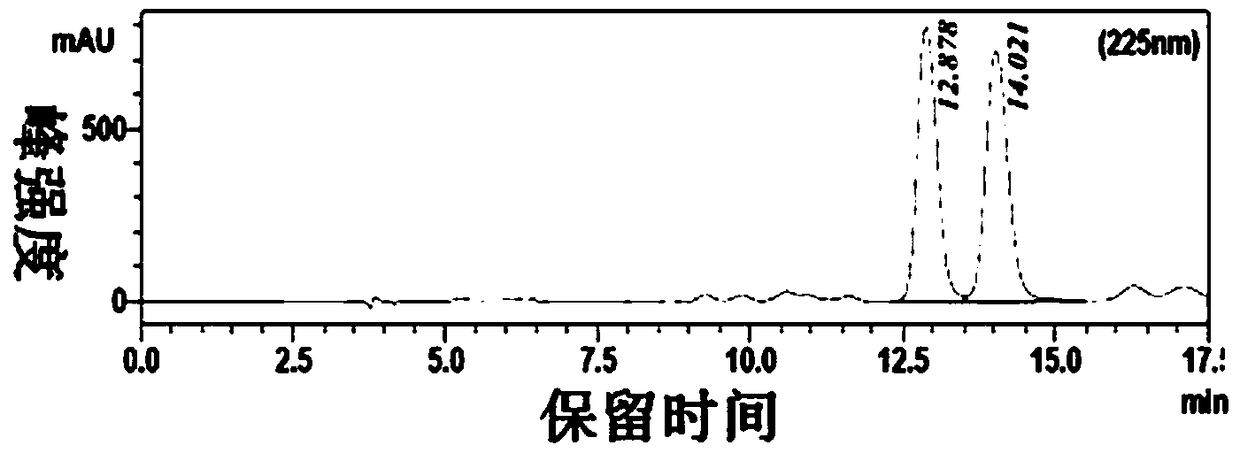
Method for determining content of three components comprising phenylephrine hydrochloride, chlorphenamine maleate and ibuprofen in compound cold treatment tablet
ActiveCN104251889AComprehensive quality inspection indicatorsGood repeatabilityComponent separationChlorphenamine maleateSulfonate
he invention discloses a method for simultaneously determining phenylephrine hydrochloride, chlorphenamine maleate and ibuprofen in a compound cold treatment medicine. The method comprises the following steps: respectively preparing a phenylephrine hydrochloride reference substance solution, a chlorphenamine maleate reference substance solution and an ibuprofen reference substance solution; preparing a compound cold treatment medicine sample solution; and determining through high performance liquid chromatography, wherein octadecyl silane-bonded silica gel (2504.0mm, 5mum) is used as a filler, a sodium octane sulfonate solution is used as a mobile phase A, acetonitrile is used as a mobile phase B, gradient elution is carried out, the column temperature is 35DEG C, the flow velocity is 1ml / min, and the detection wavelength is 264nm.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
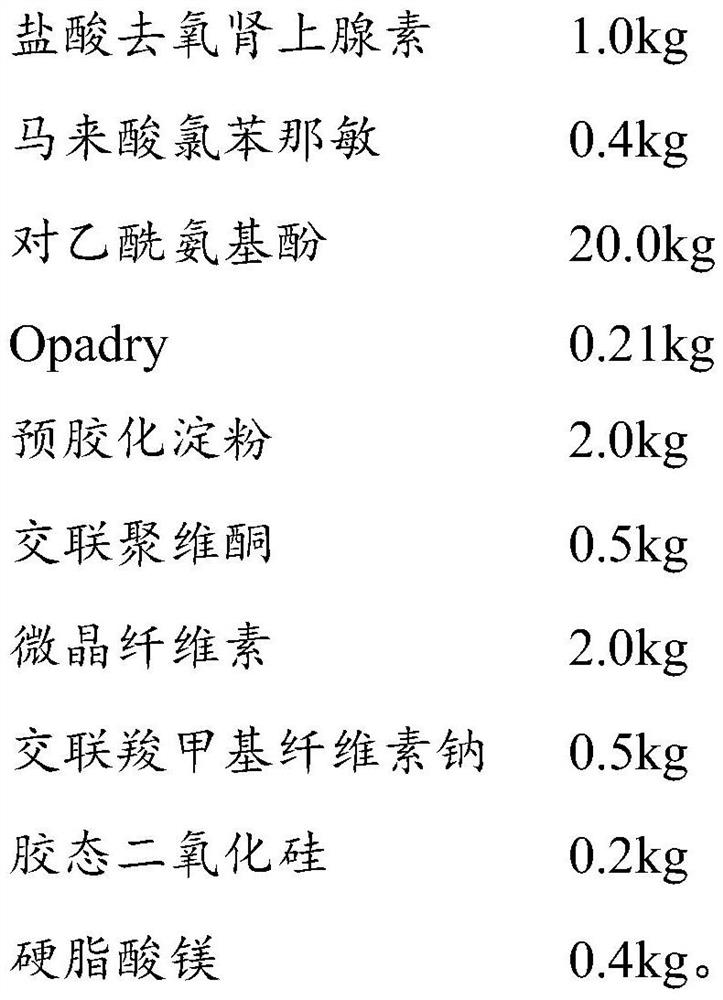
Solid preparation of compound ammonia phenol renin medicine composition liposome
The invention discloses a solid preparation of a compound ammonia phenol renin medicine composition liposome and a preparation method thereof. The method comprises that the compound ammonia phenol renin medicine composition liposome is prepared by acetaminophen, anhydrous caffein, phenylephrine hydrochloride, chlorpheniramine maleate, vitamin B1, egg yolk lecithin acyl serine, phosphatidyl ethanolamine and octadecylamine which are selected according to specified weight ratio, and then the solid preparation is prepared by the compound ammonia phenol renin medicine composition liposome through an ordinary preparation method. The solid preparation of the liposome is high in encapsulation and even in particle size, improves quality of a preparation product, reduces toxic and side effects, andis suitable for industrialized production. In addition, the preparation method is simple, and the medicine is reserved in blood circulation for a long time.
Owner:HAINAN MEIDA PHARMA
Phenylephrine ketorolac solution and preparation method
InactiveCN104856990AImprove securityAchieve superimposed effectOrganic active ingredientsSenses disorderPreservative freeSide effect
The invention relates to phenylephrine ketorolac solution and a preparation method, and provides intraocular operation washing concentrated solution without a preservative, an antioxidant and a buffer system. The phenylephrine ketorolac solution is capable of preventing the intraoperative miosis and relieving the postoperation pain, and avoiding the side effects caused by the preservative, the antioxidant and the buffer system.
Owner:WUHAN WUYAO SCI & TECH
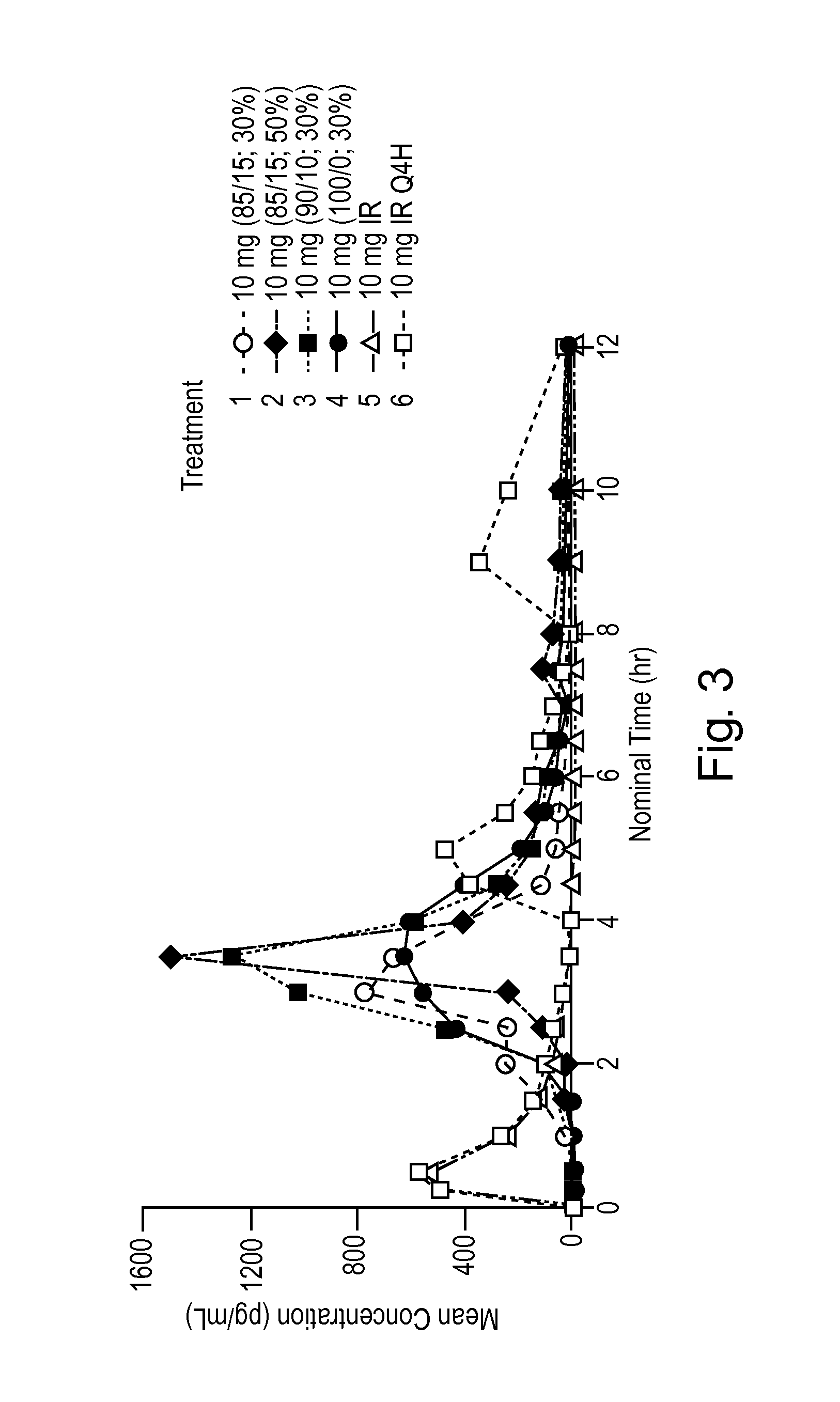
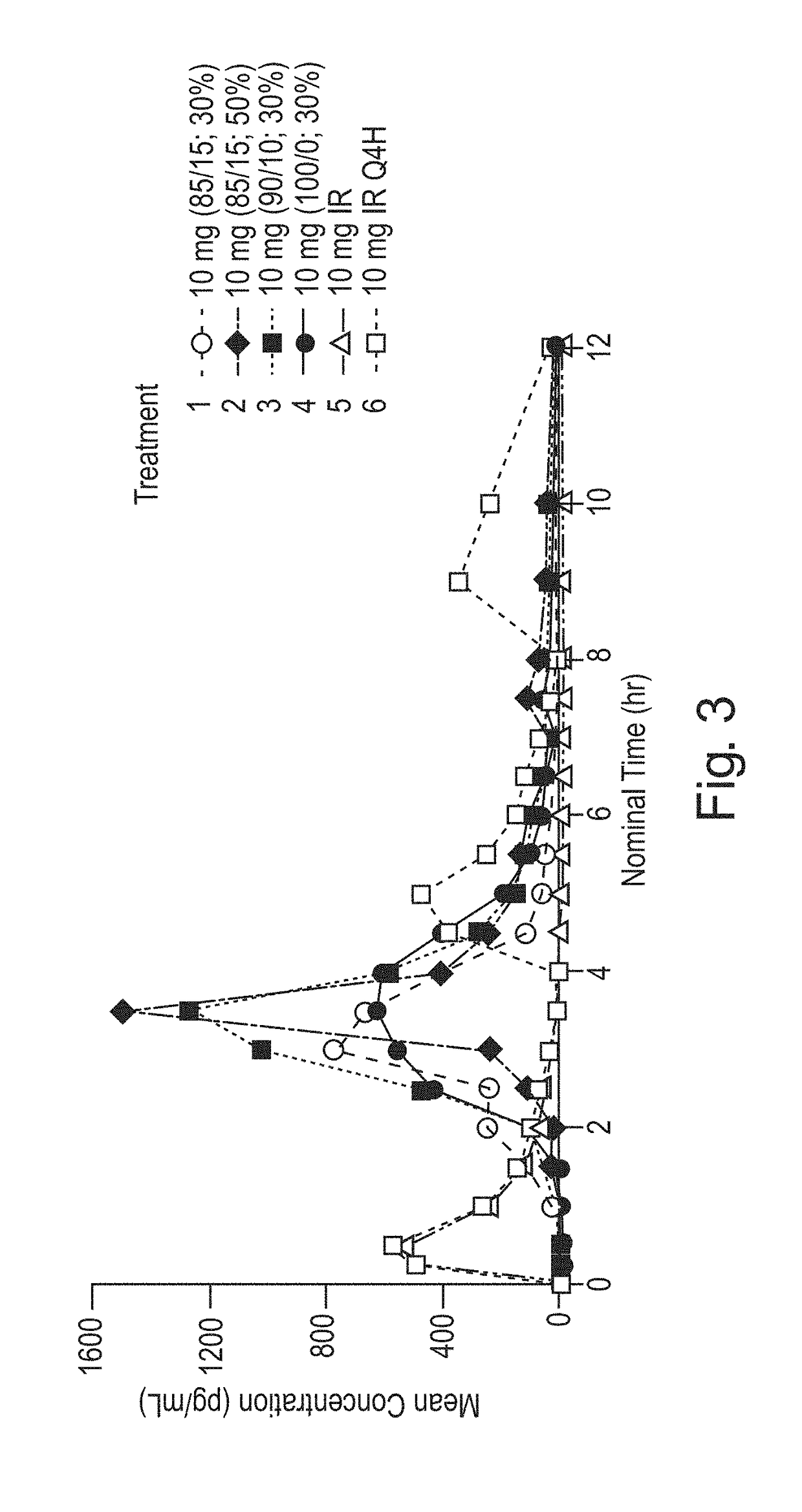
Pulsed Release Phenylephrine Dosage Forms
A multi-particle dosage form that can deliver phenylephrine in controlled pulsed doses. The dosage form can contain an immediate release form that can contain phenylephrine or a salt thereof and a plurality of delayed release particles with a coating that can contain phenylephrine or salt thereof and a pH sensitive coating.
Owner:THE PROCTER & GAMBLE COMPANY
Ketoreductase mutant for preparing R-type phenylephrine
The invention relates to a ketoreductase mutant for preparing R-type phenylephrine, and belongs to the technical field of protein engineering. Through the protein engineering, ketoreductase is subjected to saturation mutation to build a mutant library, the library is screened, and the ketoreductase mutant with chiral selectivity which is improved in the process of catalyzing alpha-chloro-3-hydroxyacetophenone into an (R)-2-Cl-1-(3-hydroxyphenyl) ethanol intermediate is obtained. The ee value of the intermediate is increased from 98.5% of a mutation template enzyme to 99.8%, and the ketoreductase mutant can be directly used for subsequent chemical reactions without being purified to directly prepare the R-type phenylephrine; and a process is further simplified, the production cost is lowered, and the ketoreductase mutant is suitable for industrial production.
Owner:SYNCOZYMES SHANGHAI
Method for determining related substances of pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride
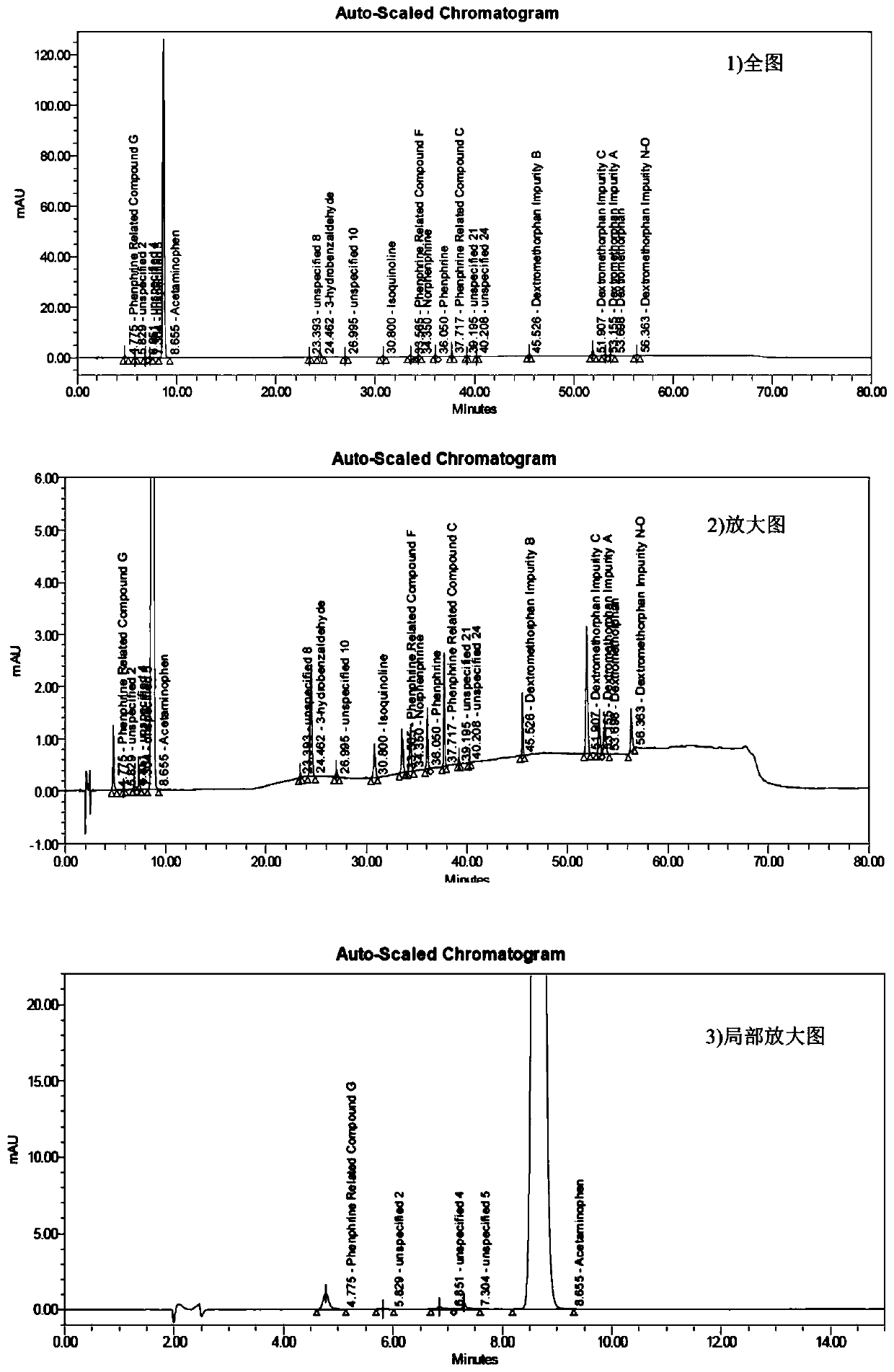
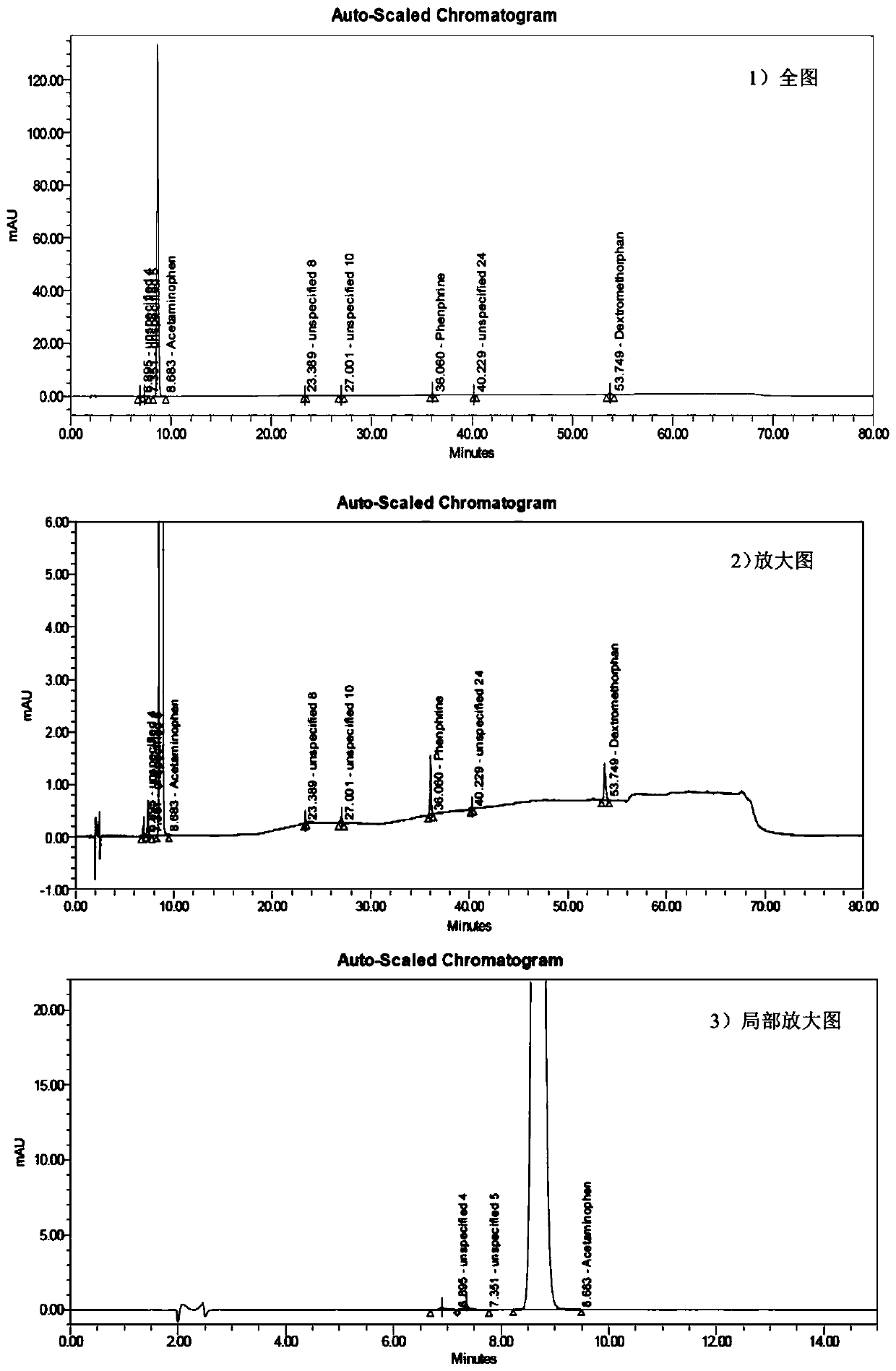
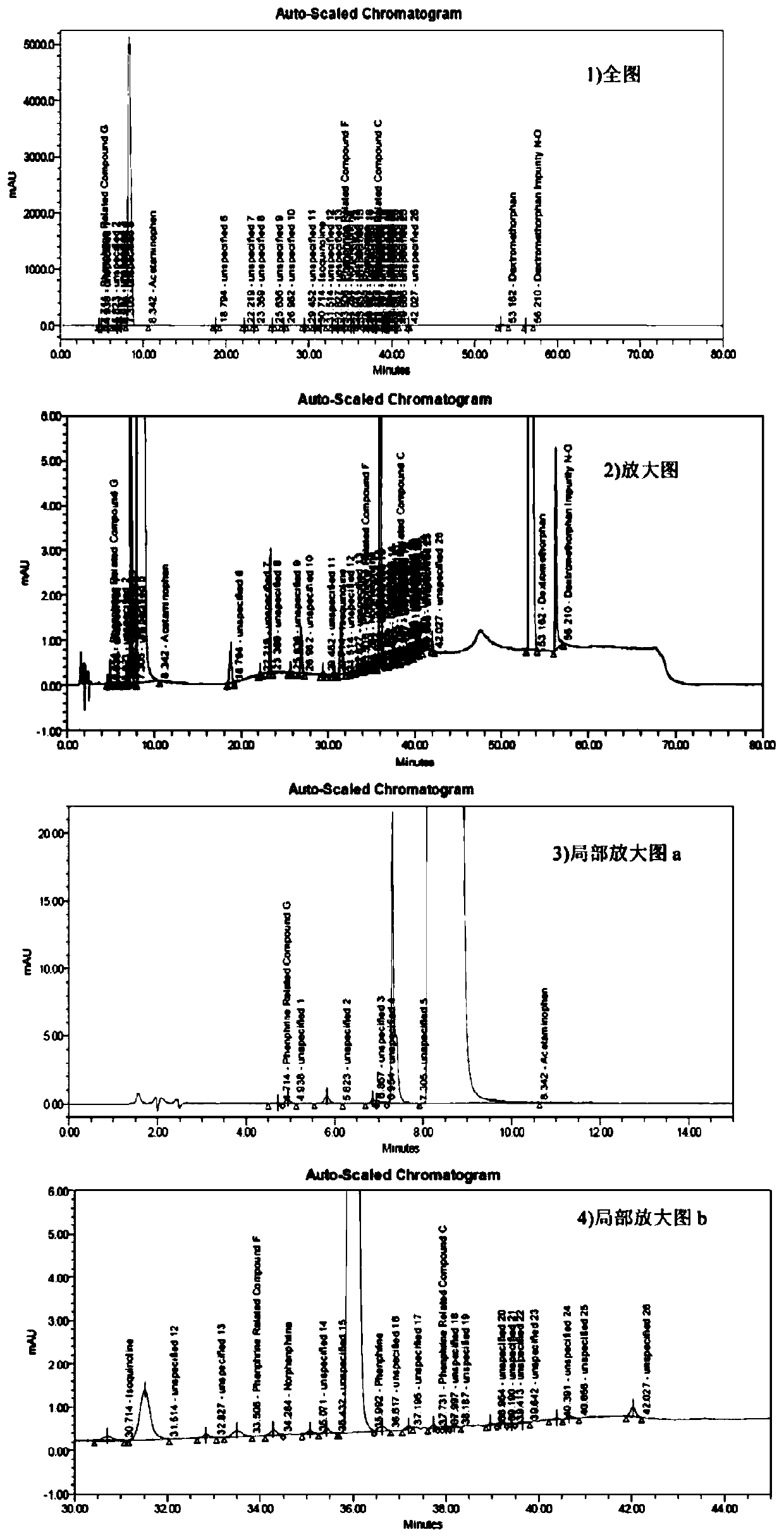
The invention discloses a method for determining related substances of a pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride. The determination method disclosed by the invention is performed by adopting a high performance liquid chromatography; proper HPLC chromatographic conditions are screened out; and 10 related substances of dextromethorphan impurities I, dextromethorphan impurities II, dextromethorphan impurities III, dextromethorphan impurities IV, norepinephrine, a phenylephrine related substance F, a 4,6 diol isoquinoline analogue, a phenylephrine related substance C, 3-hydroxybenzaldehyde and a phenylephrine related substance G in the pharmaceutical preparation of acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride can be simultaneously determined under the same condition. Effective separation of a variety of impurities can be achieved, and detection time and detection cost are greatly saved. The method can be used for quality research and quality control of pharmaceutical preparation products containing the acetaminophen, the dextromethorphan hydrobromide and the phenylephrine hydrochloride.
Owner:安士制药(中山)有限公司
Modified Release Analgesic Suspensions
ActiveUS20130142846A1Organic active ingredientsNervous disorderNon steroid anti inflammatory drugAntiinflammatory drug
A pharmaceutical dosage form comprising non-steroidal-anti-inflammatory drugs, in particular propionic acid derivatives such as ibuprofen, along with a second active ingredient having a shorter therapeutically effective plasma concentration duration, such as phenylephrine, and methods of administering the same are provided. This method provides improved therapeutic effect, in particular pain relief along with decongestant relief, over extended time periods.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Phenylephrine resinate particles having good auc
InactiveUS20140271892A1Maintaining sustained bioavailabilityPowder deliveryOrganic active ingredientsMedicineSemi solid
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
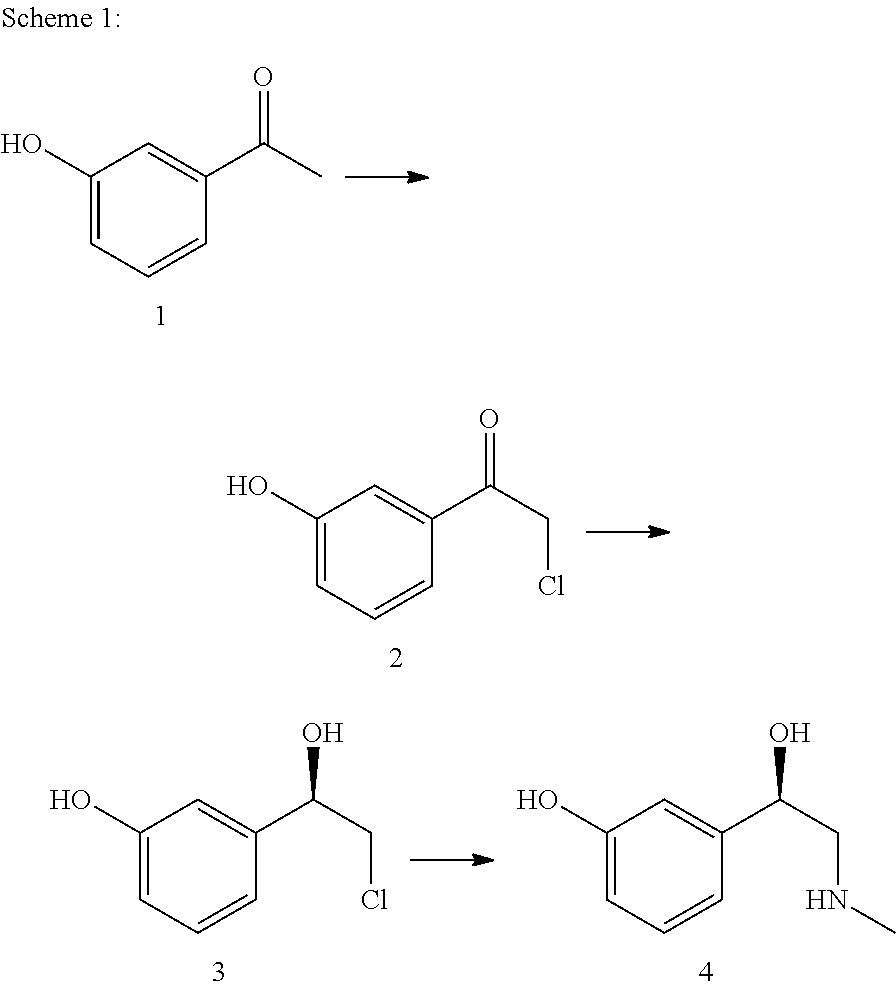
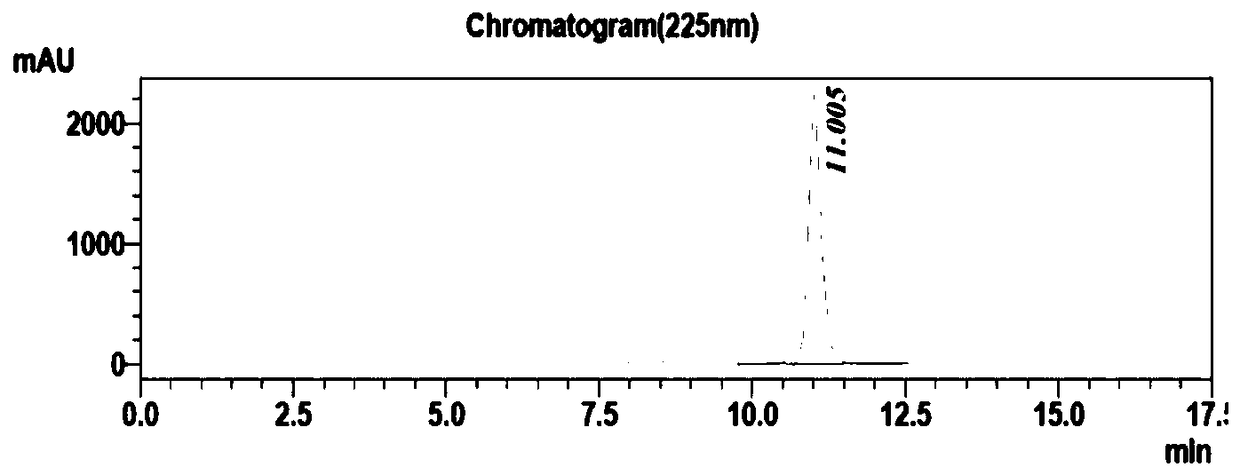
Preparation method of phenylephrine hydrochloride impurity
ActiveCN103553942AOrganic compound preparationAmino-hyroxy compound preparationPhenylephrine HydrochlorideNMR - Nuclear magnetic resonance
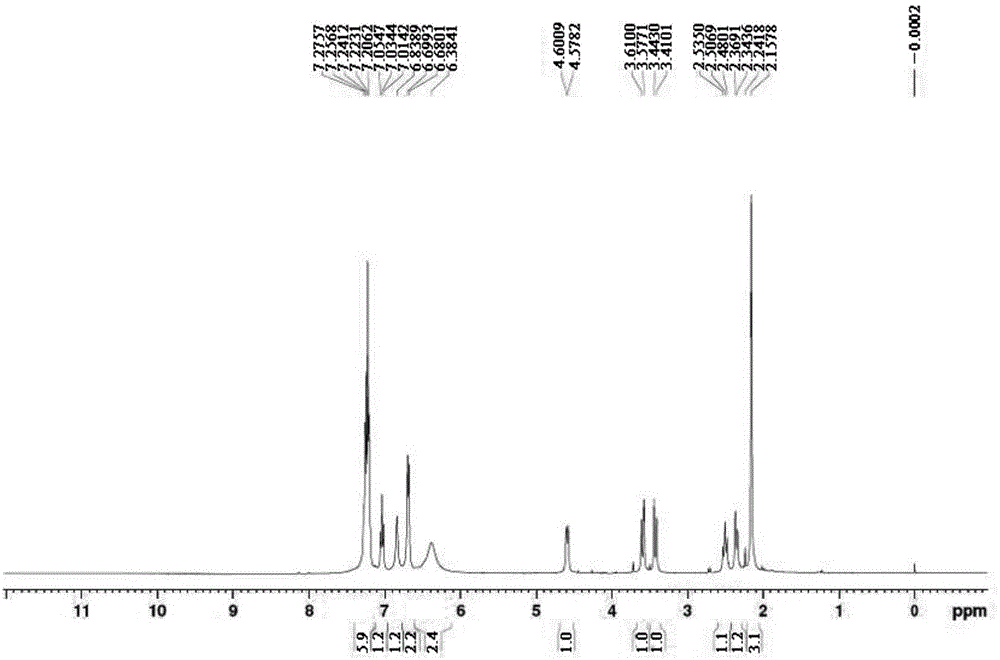
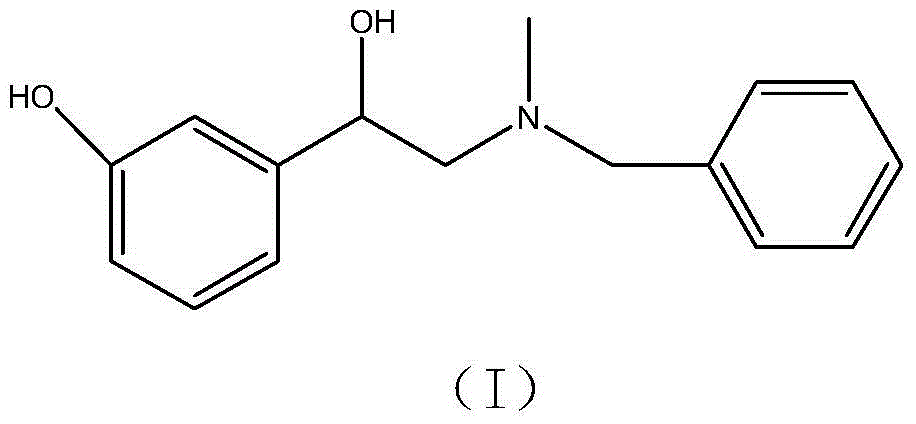
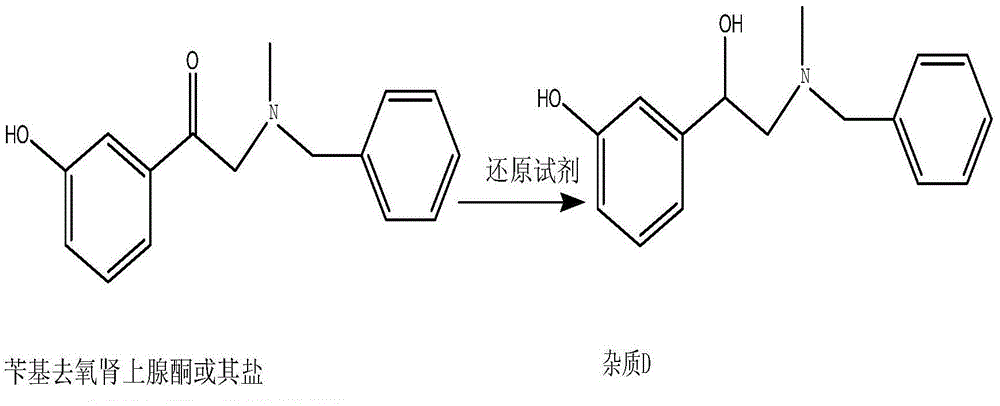
The invention belongs to the field of pharmaceutical chemistry and discloses a phenylephrine hydrochloride impurity D, namely 2-(N-benzyl methylamino)-1-(3-hydroxyl phenyl) alcohol and a preparation method thereof. An impurity D is one of main impurities of crude drugs of phenylephrine hydrochloride; the phenylephrine hydrochloride impurity D is synthesized, a reference substance is provided for examination and quantitative and qualitative analysis of the phenylephrine hydrochloride impurity, so that the quality standards of phenylephrine hydrochloride are improved, and guidance is provided for safe medication of phenylephrine hydrochloride; meanwhile, an effective test basis is provided for obtaining phenylephrine hydrochloride which satisfies EP (European Pharmacopeia) quality standards; the figure 1 is an impurity D H-nuclear magnetic resonance spectrogram.
Owner:WUHAN WUYAO SCI & TECH
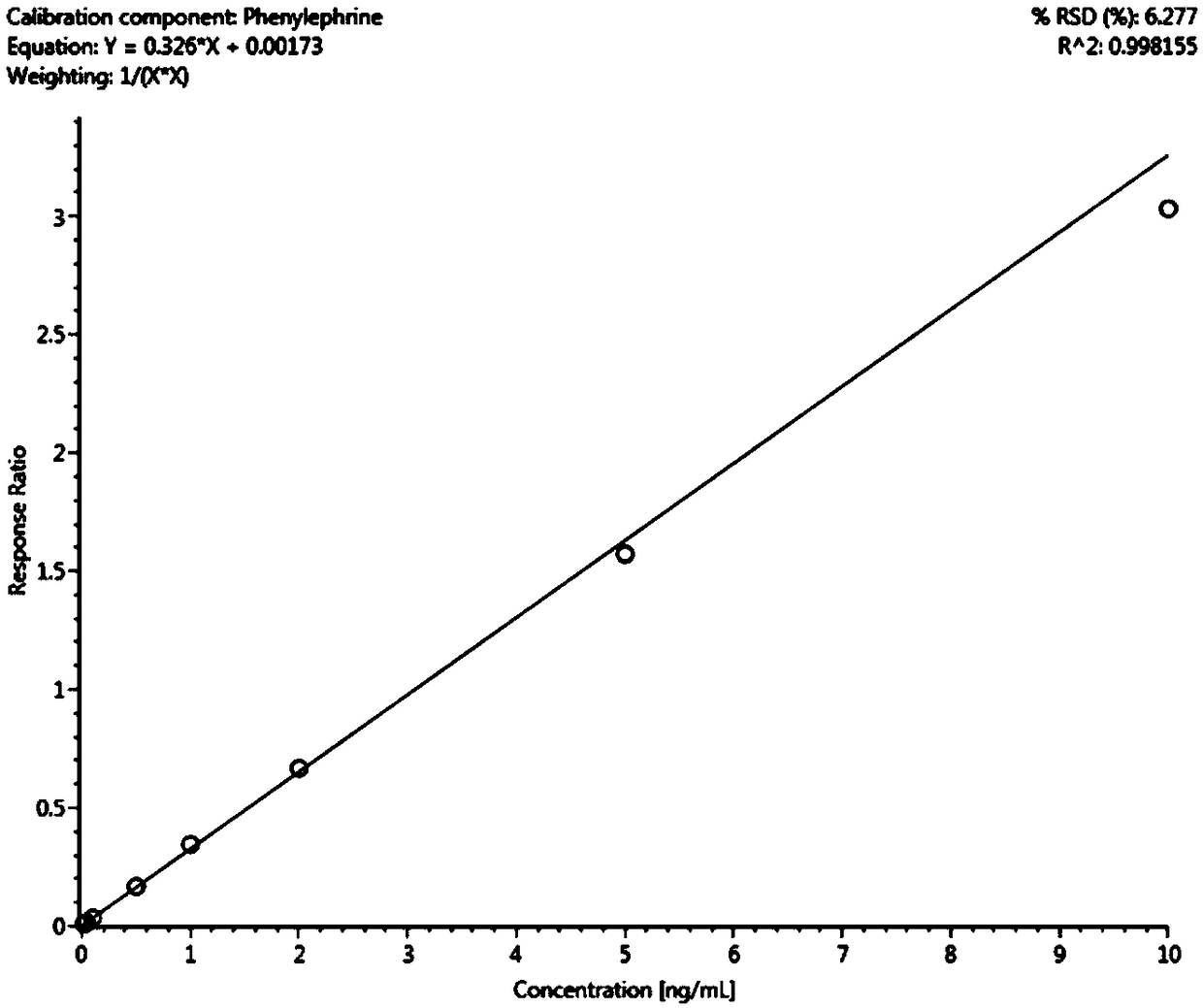
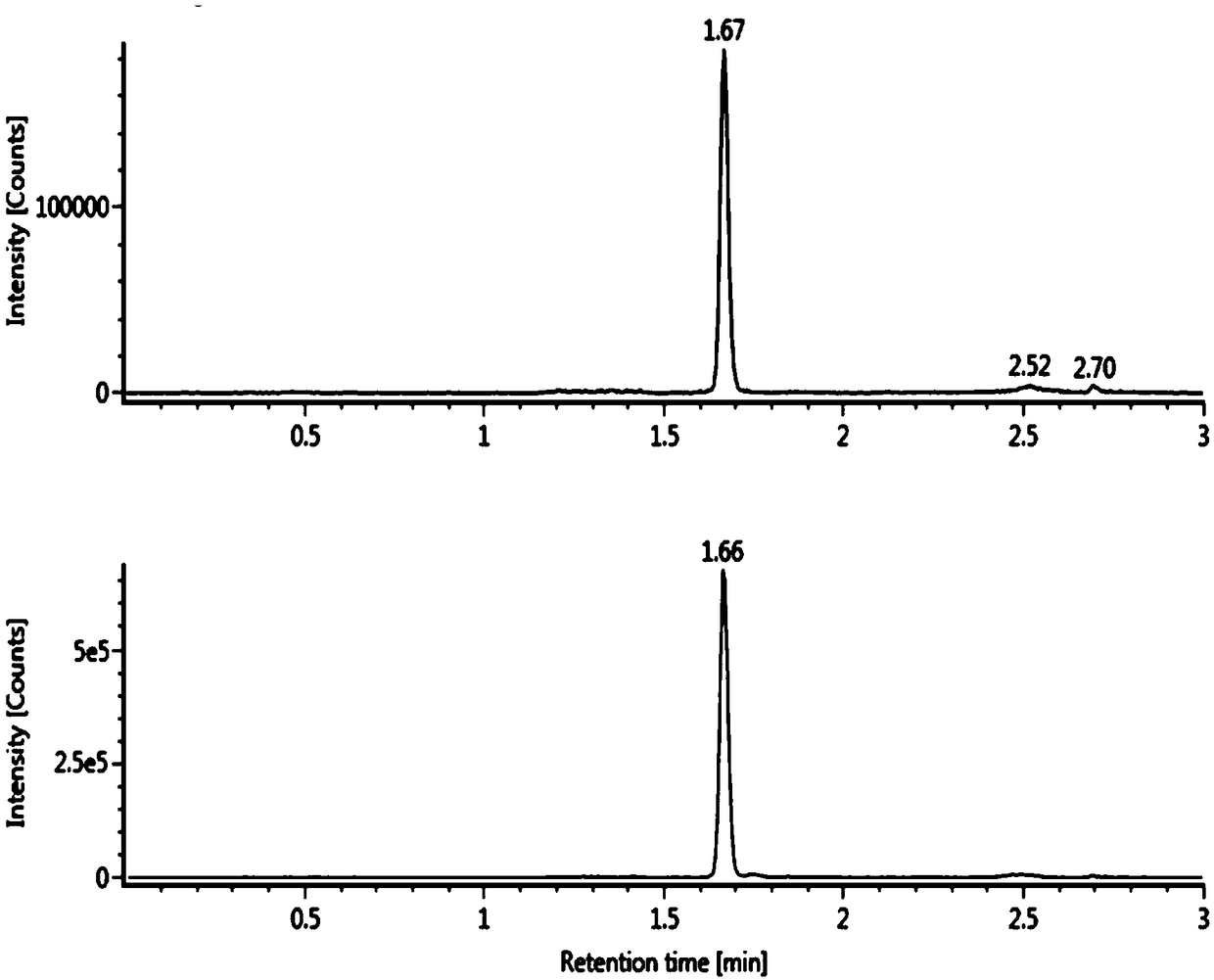
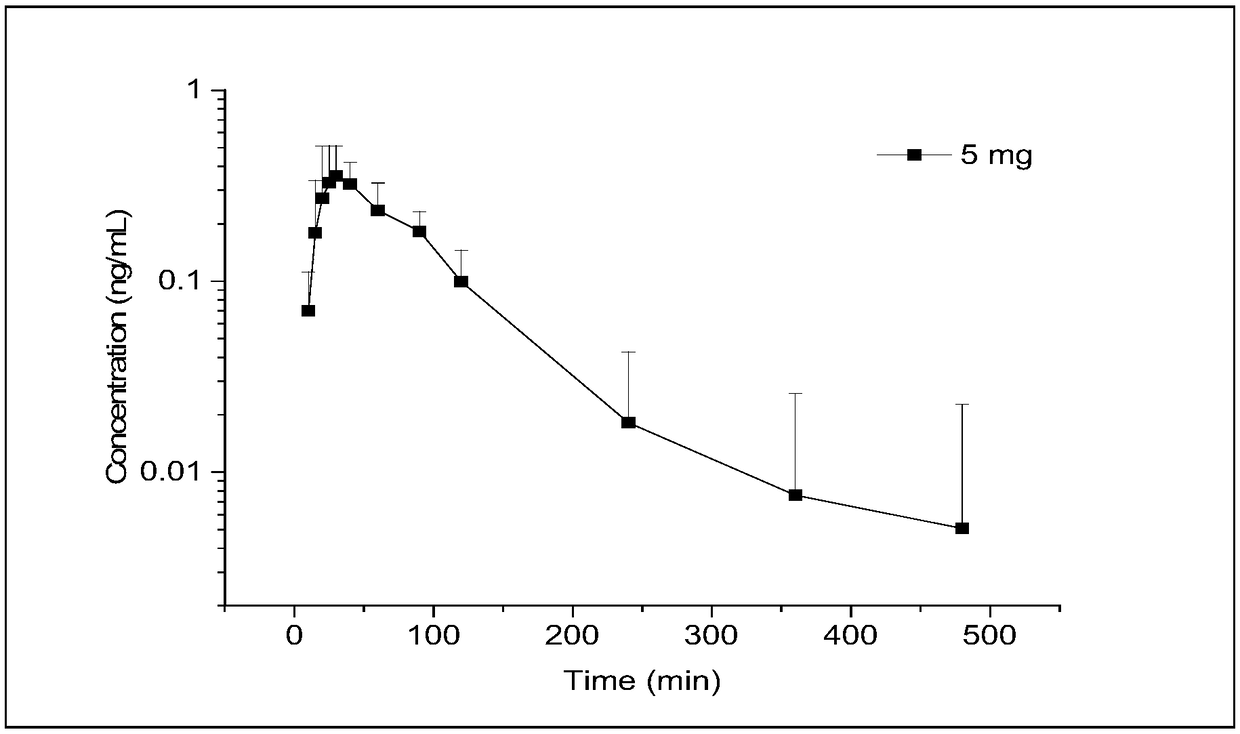
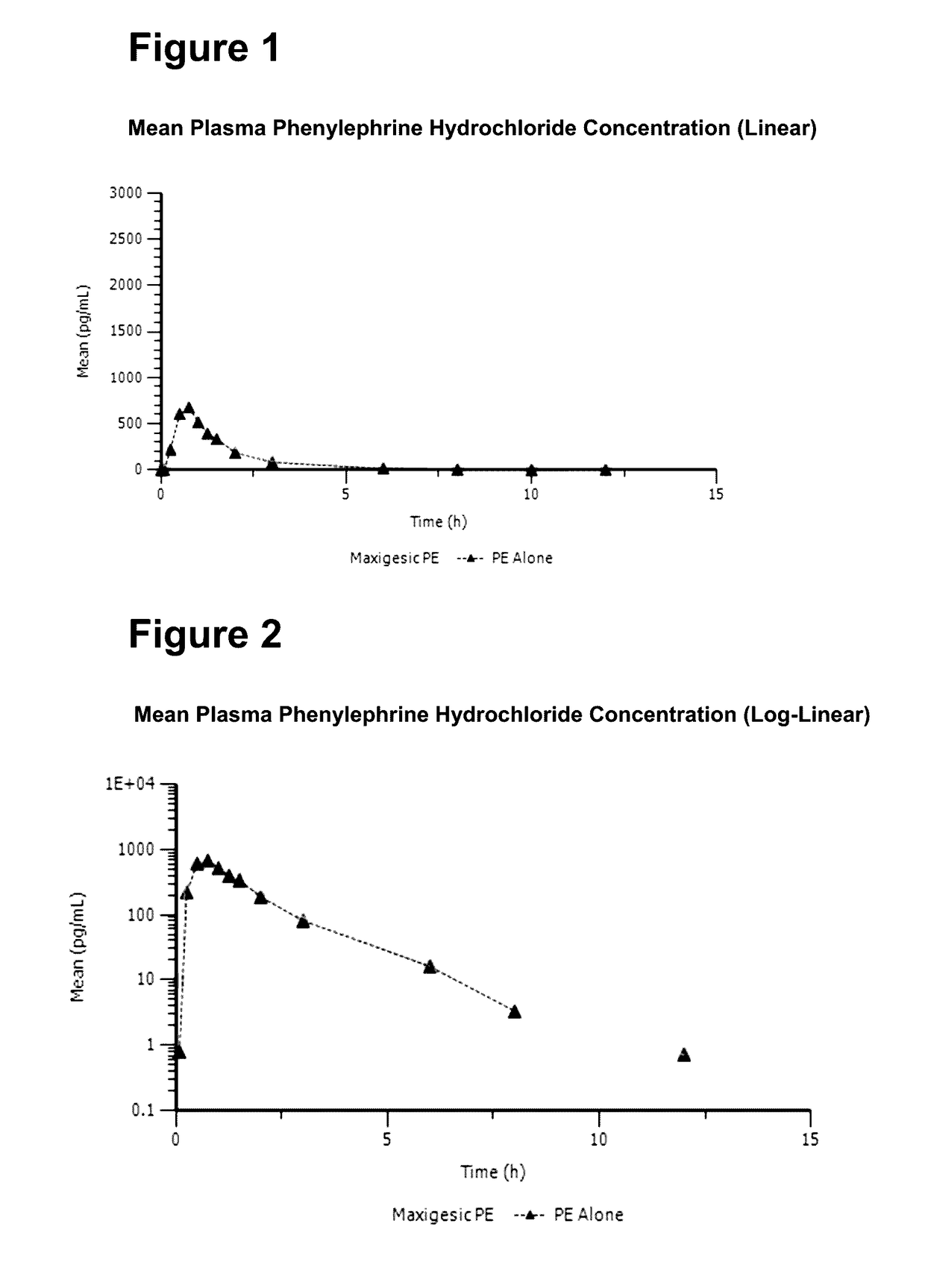
Method for measuring concentration of phenylephrine in plasma by LC-MS/MS, and pretreatment method of sample
ActiveCN109187832AImprove precipitation effectImprove stabilityComponent separationDansyl chloridePretreatment method
The invention relates to the technical field of medical detection, and specifically relates to a method for measuring the concentration of phenylephrine in plasma by LC-MS / MS. The method comprises thefollowing steps: primary extraction: adding an internal standard working solution and an extracting solution into a sample, centrifugally layering the solution after mixing the solutions uniformly, and collecting the supernatant to obtain first supernatant; derivatization reaction: concentrating and drying the first supernatant, adding a NaHCO3 buffer salt for dissolution, and then adding an acetonitrile solution of dansyl chloride to perform a derivatization reaction; and secondary extraction: adding the extracting solution into the reaction solution after the derivatization reaction for secondary extraction, centrifuging to obtain second supernatant, concentrating and drying the second supernatant, and redissolving the product to obtain a sample solution. By adoption of the pretreatment method of the sample provided by the invention, the extraction rate of low-level phenylephrine can be maximally improved, the interference of a plasma matrix is effectively removed, the specificityis improved, the minimum detection limit is reduced to 0.02ng / ml, and the sensitivity is increased to 0.1nM to adapt to the detection requirements of the concentration of the low-concentration phenylephrine in drug clinical studies.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
Medicament
Owner:AFT PHARM LTD
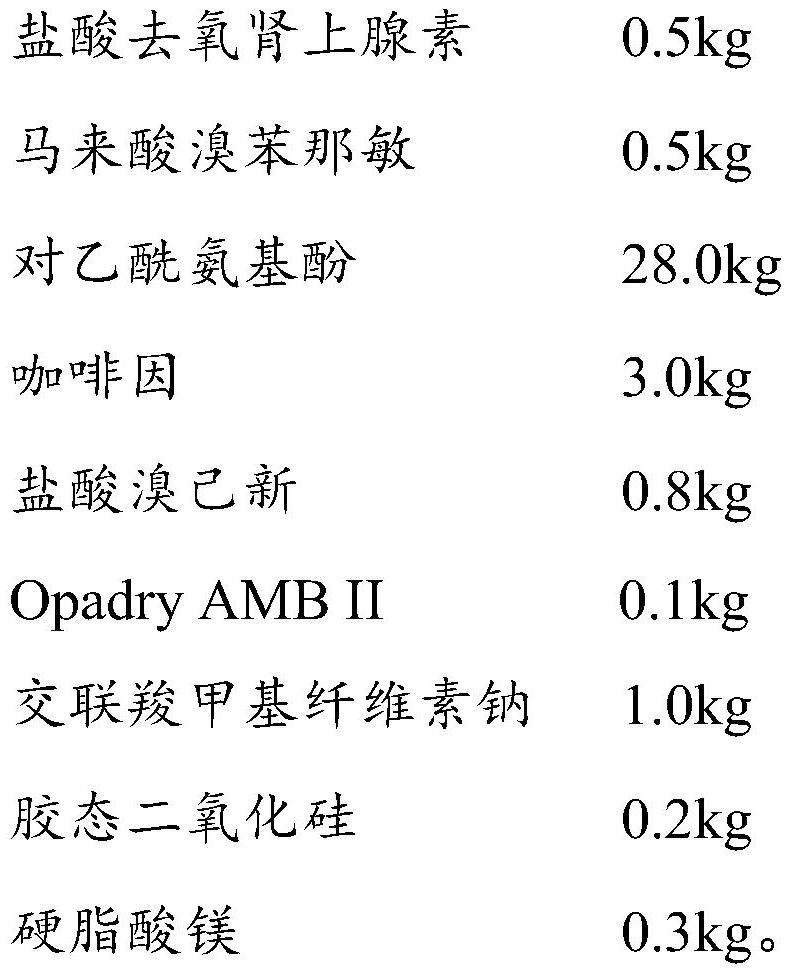
Phenylephrine hydrochloride and chlorphenamine maleate preparation and preparation method thereof
InactiveCN112472677ASolve the technical problem of impurities generated by addition reactionOrganic active ingredientsPharmaceutical non-active ingredientsChlorobenzeneActive agent
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a phenylephrine hydrochloride and chlorphenamine maleate preparation and a preparation method thereof. The phenylephrine hydrochloride and chlorphenamine maleate preparation comprises a mixture formed by a first coating and a second coating, wherein the first coating is composed of a first coreand a first coating film coating the surface of the first core, the second coating is composed of a second core and a second coating film coating the surface of the second core, the first core is a mixture of phenylephrine or a pharmaceutically acceptable salt thereof and a first pharmaceutical active agent and / or a first auxiliary material, and the second core is a mixture of chlorpheniramine maleate and / or bromopheniramine maleate and a second pharmaceutical active agent and / or a second auxiliary material. According to the phenylephrine hydrochloride and chlorphenamine maleate preparation,the first core and the second core are not in contact with each other, so that the phenylephrine is thoroughly isolated from chlorpheniramine maleate and / or bromopheniramine maleate, and the obtainedphenylephrine hydrochloride and chlorphenamine maleate preparation has higher product stability, safety and effectiveness.
Owner:BRIGHT FUTURE PHARMA LAB LTD (CN)
Novel pharmaceutical composition and its use in a method for treatment of patients with upper respiratory mucosal congestion
The present invention relates to a pharmaceutical composition including loratadine or a pharmaceutically acceptable form thereof, and phenylephrine or a pharmaceutically acceptable form thereof, for treating upper respiratory / mucosal congestion, optionally by administering to a patient four times a day.
Owner:AFT PHARM LTD
Pulsed release phenylephrine dosage forms
Owner:PROCTER & GAMBLE CO
Composition for the treatment of covid-19
A formulation provides a unique synergistic composition for the treatment of the novel Coronavirus (COVID-19). Due to multiple mechanisms and synergy of action of the ingredients involved, using drug and nutrient therapy to support the body's natural immune response, it can provide a significant advantage to currently used therapies and may also be administered prophylactically. In its optimal embodiment the composition can be prepared in solid (tablet or capsule) or liquid form, containing Aspirin or any natural salicinoid, Phenylephrine, Promethazine, Vitamin D, Vitamin C, Niacinamide, Iodine Zinc and Selenium.
Owner:LALVANI KARTAR SINGH +2
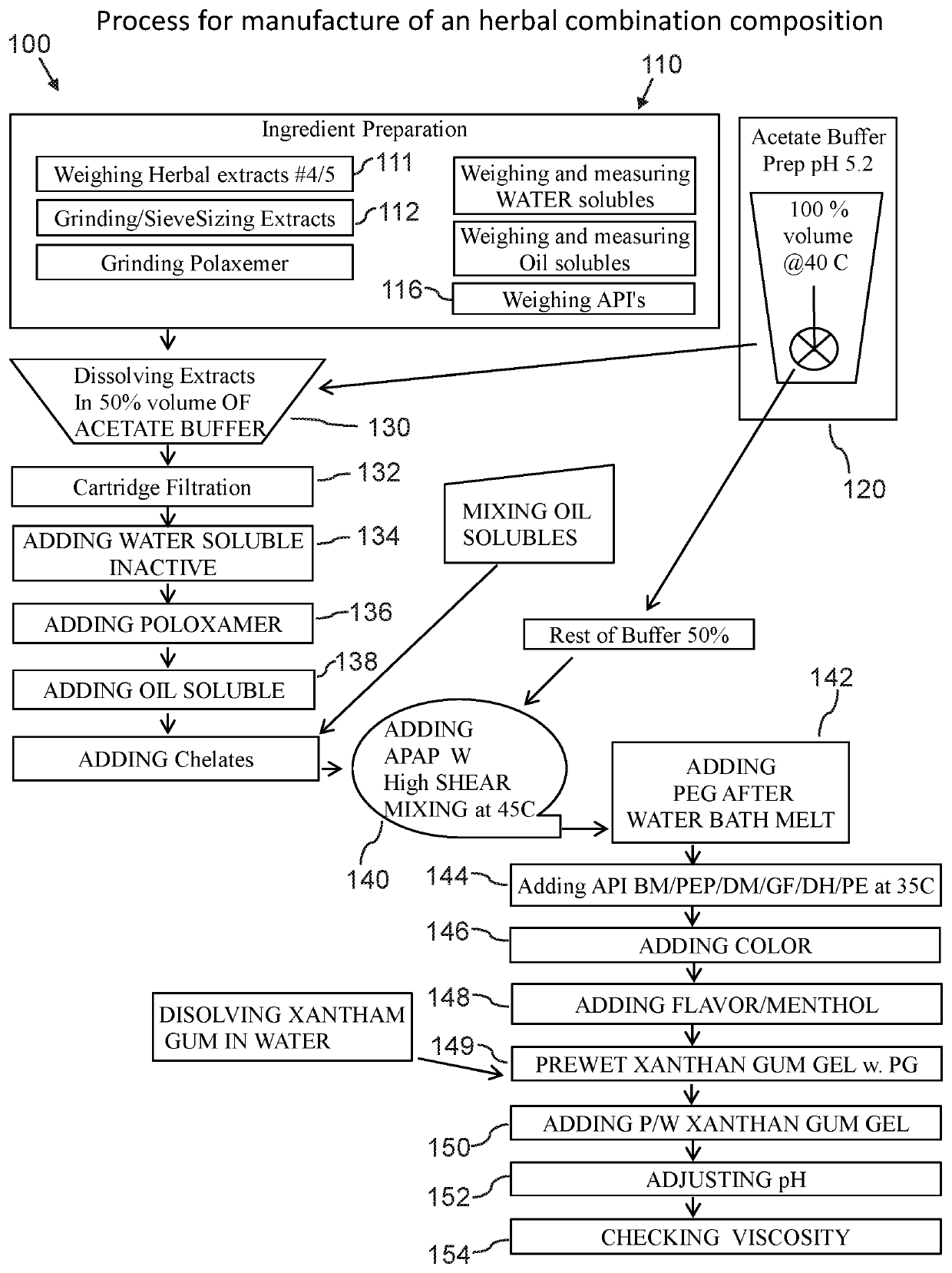
Combined herbal and pharmaceutical composition and method
ActiveUS10610559B2Short retention timeHigh resolutionEther/acetal active ingredientsPlant ingredientsBenzoic acidPolyethylene glycol
An herbal combination composition can include, herbal extracts, including combinations of: Centella asiatica, licorice, Hyssopus officinalis, Zingiber officinale, Viola odorata, Ziziphus jujuba, Chamomile, and Ocimum tenuiflorum; pharmaceutical compositions, including combinations of: Brompheniramine Maleate, Pseudoephedrine, dextromethorphan, guaifenesin, acetaminophen, phenylephrine, diphenhydramine. The herbal combination composition can further include: polyethylene glycol; propylene glycol; poloxamer 407; ethylenediaminetetraacetic acid; methyl paraben; potassium sorbate; propyl paraben; xanthan gum; sodium citrate, citric acid; anhydrous citric acid; and purified acetate buffered water. Also disclosed is a method for manufacture of an herbal combination composition, including dissolving herbal extracts, adding poloxamer, adding pharmaceutical compositions, adding acetate buffer, adding xanthan gum gel, adding acetate buffer.
Owner:SYED UWAIS M
Phenylephrine formulations with improved stability
A pharmaceutical composition includes a pharmaceutical polysaccharide and phenylephrine hydrochloride. The ratio of said polysaccharide to phenylephrine hydrochloride is sufficient to dilute the composition such that phenylephrine hydrochloride is stable at high temperature and humidity.
Owner:GLAXOSMITHKLINE CONSUMER HEALTHCARE GMBH & CO KG
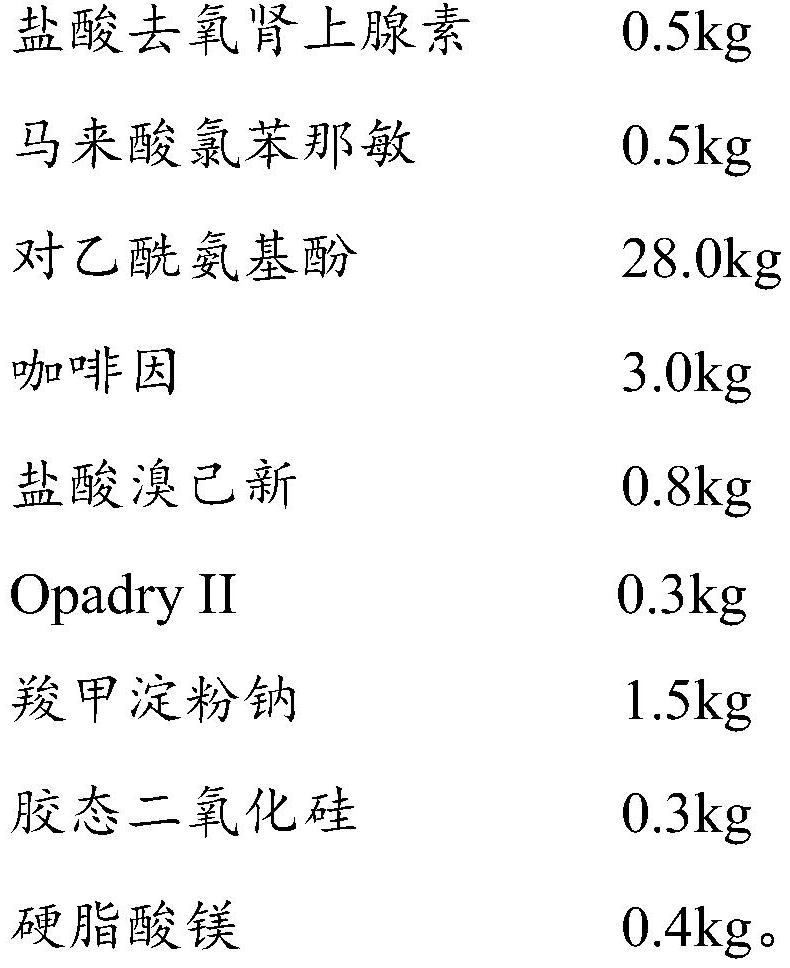
Phenylephrine hydrochloride and chlorphenamine maleate preparation and preparation method thereof
InactiveCN112472678AAvoid reactionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsChlorobenzenePharmaceutical medicine
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a phenylephrine hydrochloride and chlorphenamine maleate preparation and a preparation method thereof. The phenylephrine hydrochloride and chlorphenamine maleate preparation comprises a mixture formed by a first coating and a second coating, wherein the first coating is composed of a first coreand a first coating film coating the surface of the first core, the second coating is composed of a second core and a second coating film coating the surface of the second core, the first core is phenylephrine or a pharmaceutically acceptable salt thereof, and the second core is chlorpheniramine maleate and / or bromopheniramine maleate. According to the phenylephrine hydrochloride and chlorphenamine maleate preparation disclosed by the invention, the first core and the second core are not in contact with each other, so that thorough isolation of phenylephrine from chlorpheniramine maleate and / or bromopheniramine maleate is realized, secondary amino groups on phenylephrine are prevented from reacting with maleate radicals, the obtained phenylephrine hydrochloride and chlorphenamine maleatepreparation has higher product stability, and the safety and effectiveness of clinical medication are improved.
Owner:BRIGHT FUTURE PHARMA LAB LTD (CN)
Phenylephrine Hydrochloride Compositions and Containers
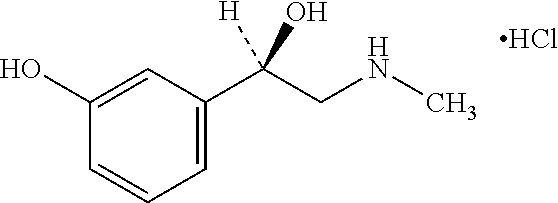
PendingUS20210228507A1Enhance and preserve chemical stabilityExtended shelf lifeOrganic active ingredientsInorganic non-active ingredientsNorphenylephrinePharmacology
A ready-to-administer antioxidant free phenylephrine compositions has improved stability and is optionally free of metal chelating agents. Contemplated compositions are preferably packaged into a flexible polymer bag and maintain degradation of the phenylephrine at remarkably low levels, even over extended storage periods.
Owner:ENDO VENTURES LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com