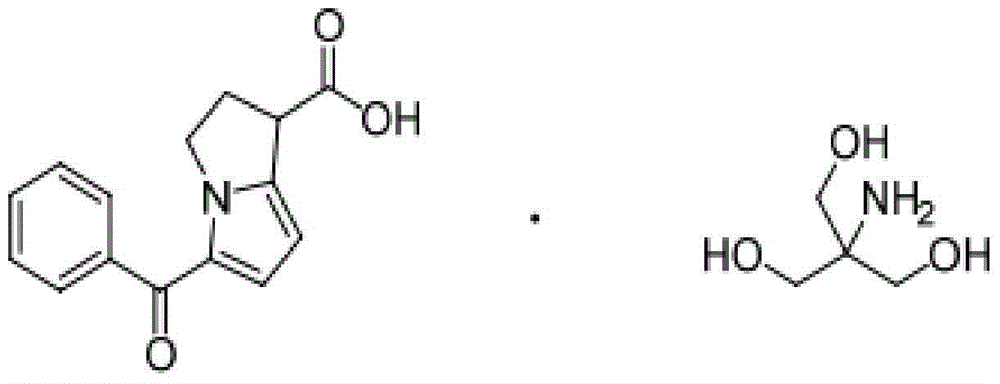
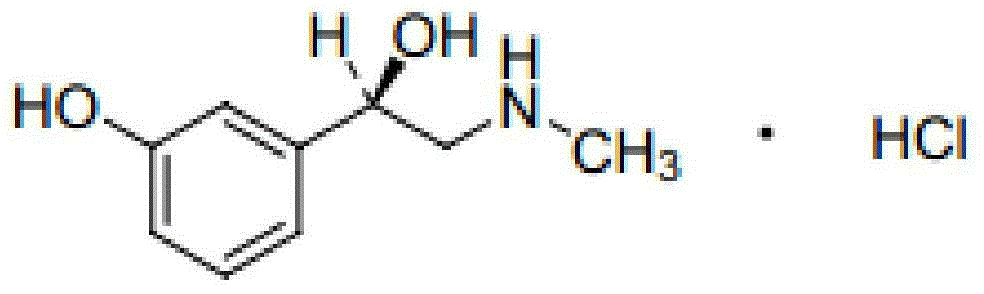Phenylephrine ketorolac solution and preparation method
A technology of phenylephrine and phenylephrine hydrochloride, which is used in pharmaceutical formulations, drug delivery, sensory diseases, etc., to relieve postoperative pain, increase safety, and prevent intraoperative pupil dilation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: One of the preparations of phenylephrine ketorolac solution
[0034] (4ml: phenylephrine 40.64mg and ketorolac 11.52mg)
[0035]
[0036] Remarks: Phenylephrine hydrochloride is fed at 100%, and ketorolac tromethamine is fed at 104%.
[0037] Preparation process: Weigh 49.6 g of phenylephrine hydrochloride and 17.64 g of ketorolac tromethamine according to the prescription amount, add them to water for injection at 50°C, and stir to dissolve completely. Use 0.05% (w / v) activated carbon for needles and stir at 50°C for 20min. Use a 0.45 μm filter membrane to filter the filtrate through a 0.22 μm filter membrane, then adjust the pH to about 6.3 with 1% (w / v) sodium hydroxide solution or 1M hydrochloric acid solution, and finally add water for injection to make up to 4000ml , intermediate detection. Nitrogen protection, filling and sealing in 5ml ampoules, 4ml / branch. 121°C, 15min high-pressure steam sterilization, leak detection, light inspection of a...
Embodiment 2
[0038] Example 2: According to the prescription in WO2014066485A1, a phenylephrine ketorolac solution was prepared.
[0039] (4ml: phenylephrine 40.64mg and ketorolac 11.52mg)
[0040]
[0041]
[0042] Remarks: Phenylephrine hydrochloride is fed at 100%, and ketorolac tromethamine is fed at 104%.
[0043] Preparation process: Weigh 49.6g of phenylephrine hydrochloride, 17.64g of ketorolac tromethamine, 0.96g of citric acid (monohydrate), and 23.53g of sodium citrate (dihydrate) according to the prescription amount, add Into the water for injection at 50°C, stirring and dissolving completely. Use 0.05% (w / v) activated carbon for needles and stir at 50°C for 20min. Use a 0.45 μm filter membrane to filter the filtrate through a 0.22 μm filter membrane, then adjust the pH to about 6.3 with 1% (w / v) sodium hydroxide solution or 1M hydrochloric acid solution, and finally add water for injection to make up to 4000ml , intermediate detection. Nitrogen protection, filling an...
Embodiment 3
[0044] Embodiment 3: in the preparation process of embodiment 1 and embodiment 2, we have investigated pH value. And with reference to the drug stability guidelines, the samples prepared in Example 1 and Example 2 were respectively investigated for influencing factors, placed under the conditions of a temperature of 60 ° C and a light of 4500 ± 500 lx, and samples were taken out on the 5th and 10th days respectively Perform a pH test. The results are shown in Table 1.
[0045] Table 1 Changes in pH during the preparation process and influencing factors of self-developed and patented prescriptions
[0046]
[0047] The test results show that the pH values of the self-developed and patented prescriptions are relatively stable after the pH is adjusted during the preparation process and placed under the conditions of high temperature of 60°C and light of 4500±500lx for 5 days and 10 days. This proves that the self-developed prescription can have a relatively stable pH value...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com